Question
The specific heat of a gas varies linearly with absolute temperature according to the following equation: If the gas entropy at 1 bar and 273
The specific heat of a gas varies linearly with absolute temperature according to the following equation:
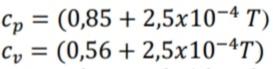
If the gas entropy at 1 bar and 273 K is zero, find the gas entropy at 25 bar and 750 K.
Note:
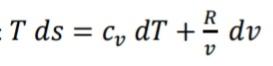
3D (0,85 + 2,510-4 T) %3D (0,56 + 2,510-4T)
Step by Step Solution
3.36 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
College Algebra
Authors: Margaret L. Lial, John Hornsby, David I. Schneider, Callie Daniels
12th edition
134697022, 9780134313795 , 978-0134697024
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



