Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The standard cell potential of the cell shown in the following diagram is +0.22 V. Ni | Ni(NO:)2(aq) ||KI(aq)| Hg2l2(s) | Hg() | Pt
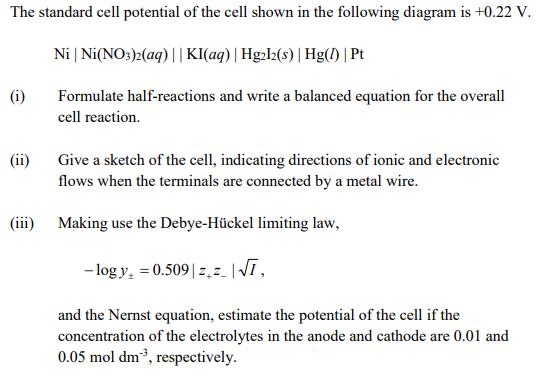
The standard cell potential of the cell shown in the following diagram is +0.22 V. Ni | Ni(NO:)2(aq) ||KI(aq)| Hg2l2(s) | Hg() | Pt (i) Formulate half-reactions and write a balanced equation for the overall cell reaction. (ii) Give a sketch of the cell, indicating directions of ionic and electronic flows when the terminals are connected by a metal wire. (iii) Making use the Debye-Hckel limiting law, - log y, = 0.509| z,z |V, and the Nernst equation, estimate the potential of the cell if the concentration of the electrolytes in the anode and cathode are 0.01 and 0.05 mol dm3, respectively.
Step by Step Solution
★★★★★
3.54 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
635f95c868869_232828.pdf
180 KBs PDF File
635f95c868869_232828.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


