Question
The standard potential of the AgCI/Ag,Cl- couple fits the expression E/V = 0.23659 - 4.8564 x 10-4 (0/C) -3.4205 x 10-6 (0/ C)2 +
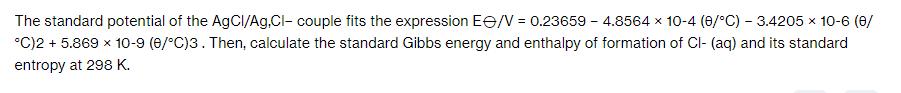
The standard potential of the AgCI/Ag,Cl- couple fits the expression E/V = 0.23659 - 4.8564 x 10-4 (0/C) -3.4205 x 10-6 (0/ C)2 + 5.869 x 10-9 (0/C)3. Then, calculate the standard Gibbs energy and enthalpy of formation of Cl- (aq) and its standard entropy at 298 K.
Step by Step Solution
3.37 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1
The detailed explanation for the answers is given below Therefore These are the required answers ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals Of Biostatistics
Authors: Bernard Rosner
8th Edition
130526892X, 978-1305465510, 1305465512, 978-1305268920
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



