Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The Stirling engine consists of two constant volume processes and two isothermal processes. Consider the cycle given below for 1.00 mol of an ideal
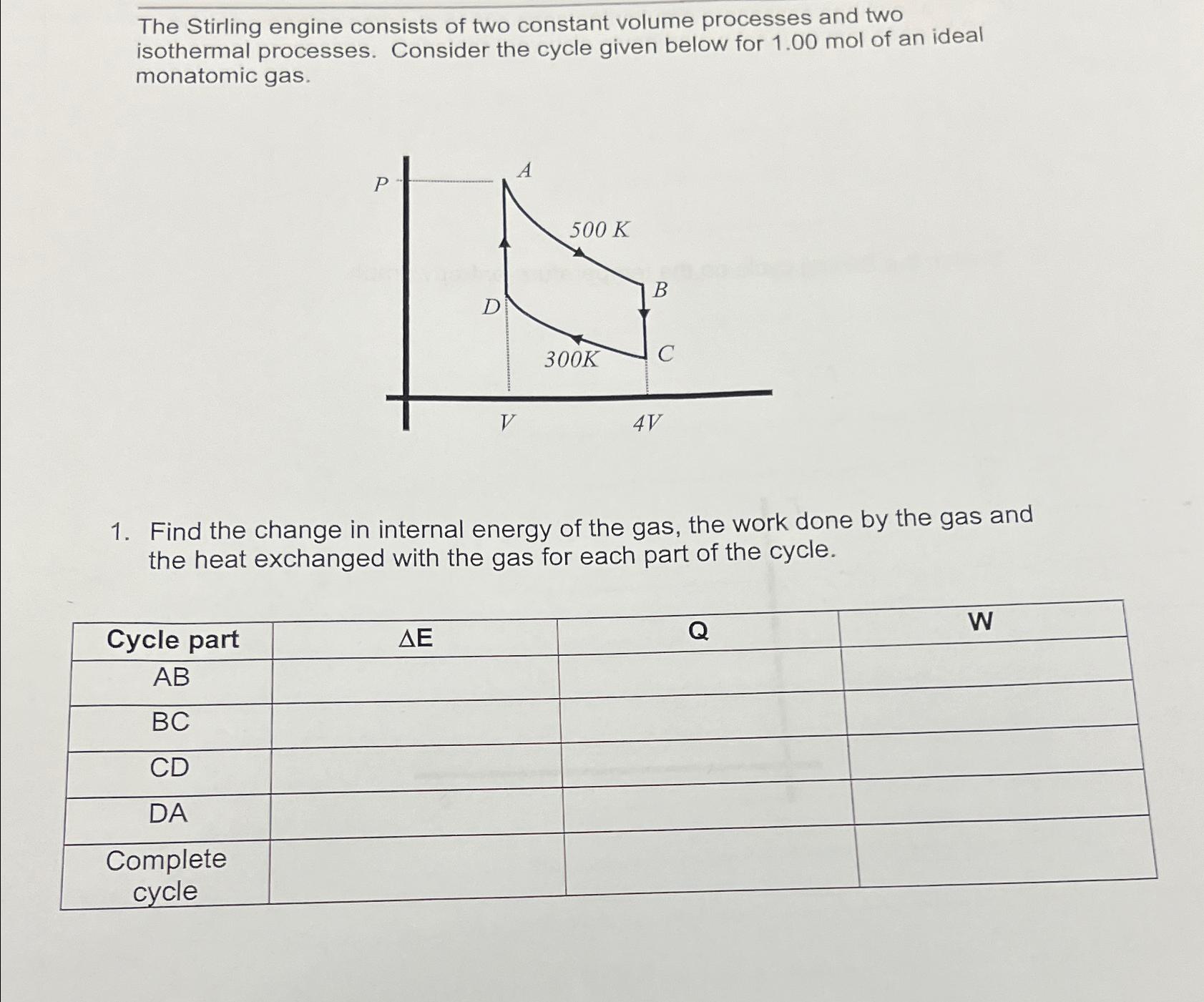
The Stirling engine consists of two constant volume processes and two isothermal processes. Consider the cycle given below for 1.00 mol of an ideal monatomic gas. A 500 K B D 300K C 4V 1. Find the change in internal energy of the gas, the work done by the gas and the heat exchanged with the gas for each part of the cycle. Cycle part AB BC CD DA Complete cycle Q W
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


