Question
The synthesis of benzoin proceeds as follows. and the following procedure is performed: A solution of sodium cyanide (1.1 g, 0.02 mol) in water (10
The synthesis of benzoin proceeds as follows.
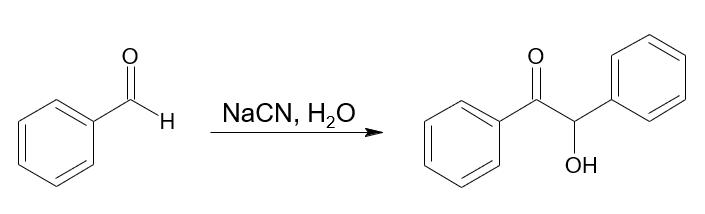
and the following procedure is performed:
A solution of sodium cyanide (1.1 g, 0.02 mol) in water (10 mL) was added to a stirred solution of benzaldehyde (10.6 g, 0.1 mol) in 15 mL ethanol. The reaction mixture was refiuxed gently for half an hour and cooled in an ice bath. The precipitated product was filtered, washed with cold water, dried well and crystallized from ethanol to afford 9.0 g (85.0%) of benzoin.
1. indicate what by-products or impurities are removed by filtration
2. indicate what by-products or impurities are removed in the recrystallization from ethanol
3. Why is the reaction mixture placed in an ice bath?
4. How can the product be dried? What methodology can be used?
Step by Step Solution
3.40 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Quantitative Chemical Analysis
Authors: Daniel C. Harris
8th edition
1429218150, 978-1429218153
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



