Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The table below shows the volumes of nitrogen (IV) oxide gas produced when different volumes of 1m nitric acid were each reacted with 2.07g
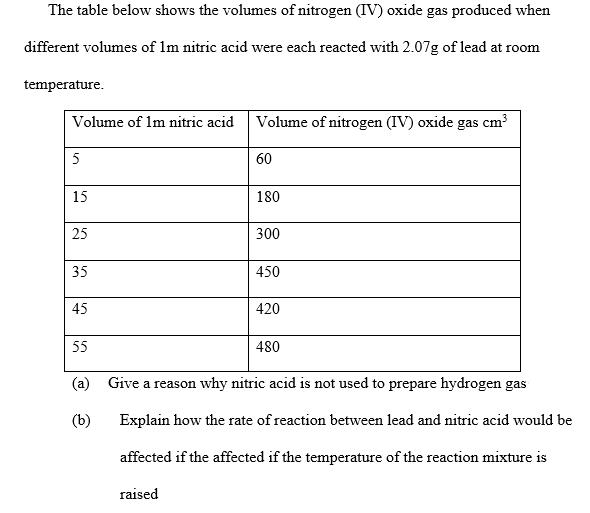
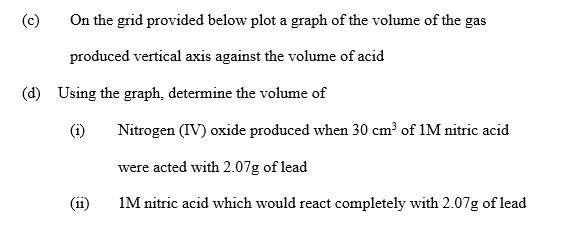

The table below shows the volumes of nitrogen (IV) oxide gas produced when different volumes of 1m nitric acid were each reacted with 2.07g of lead at room temperature. Volume of 1m nitric acid 5 15 25 35 45 55 a) (b) Volume of nitrogen (IV) oxide gas cm raised 60 180 300 450 420 480 Give a reason why nitric acid is not used to prepare hydrogen gas Explain how the rate of reaction between lead and nitric acid would be affected if the affected if the temperature of the reaction mixture is On the grid provided below plot a graph of the volume of the gas produced vertical axis against the volume of acid (d) Using the graph, determine the volume of (1) Nitrogen (IV) oxide produced when 30 cm of 1M nitric acid were acted with 2.07g of lead 1M nitric acid which would react completely with 2.07g of lead (e) Using the answer in d (ii) above determine (1) (11) The volume of 1 m nitric acid that would react with one mole of Pb/lead (Pb = 207) The volume of nitrogen (IV) oxide gas produced when one mole of lead reacts with excess 1M nitric at room temperature (f) Calculate the number of moles of (11) 1M Nitric acid that reacted with one mole of lead Nitrogen (IV) oxide produced when one molar of lead were reacted with excess nitric acid. Molar gas volume = 24000 cm (iii) Using the answer in (21) (i) and (ii) above write the equation for the reaction between lead and nitric acid given that one mole of lead nitrate and two moles of water were also produced.
Step by Step Solution
★★★★★
3.55 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
a Nitric acid HNO 3 is not typically used to prepare hydrogen gas H 2 because it is a strong oxidizi...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


