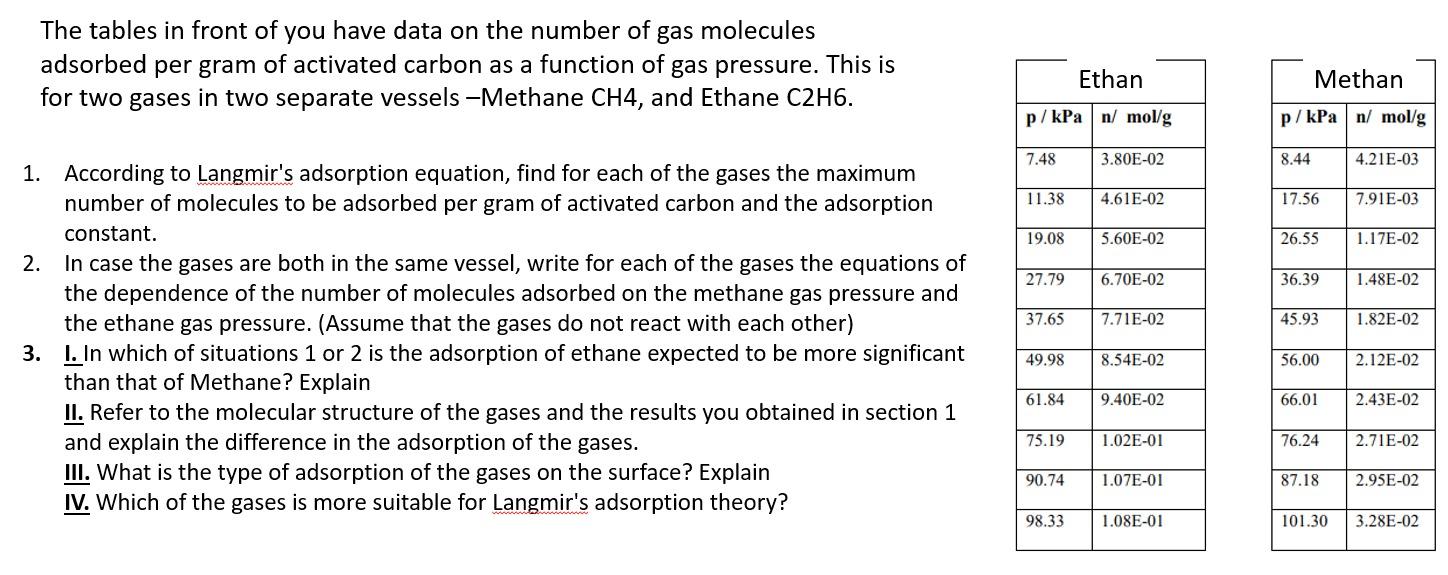
The tables in front of you have data on the number of gas molecules adsorbed per gram of activated carbon as a function of gas pressure. This is for two gases in two separate vessels -Methane CH4, and Ethane C2H6. Ethan Methan p/kPa n/ mol/g p/kPa n/ mol/g 7.48 3.80E-02 8.44 4.21E-03 11.38 4.61E-02 17.56 7.91E-03 19.08 5.60E-02 26.55 1.17E-02 27.79 6.70E-02 36.39 1.48E-02 37.65 7.71E-02 45.93 1.82E-02 1. According to Langmir's adsorption equation, find for each of the gases the maximum number of molecules to be adsorbed per gram of activated carbon and the adsorption constant. 2. In case the gases are both in the same vessel, write for each of the gases the equations of the dependence of the number of molecules adsorbed on the methane gas pressure and the ethane gas pressure. (Assume that the gases do not react with each other) 3. I. In which of situations 1 or 2 is the adsorption of ethane expected to be more significant than that of Methane? Explain II. Refer to the molecular structure of the gases and the results you obtained in section 1 and explain the difference in the adsorption of the gases. III. What is the type of adsorption of the gases on the surface? Explain IV. Which of the gases is more suitable for Langmir's adsorption theory? 49.98 8.54E-02 56.00 2.12E-02 61.84 9.40E-02 66.01 2.43E-02 75.19 1.02E-01 76.24 2.71E-02 90.74 1.07E-01 87.18 2.95E-02 98.33 1.08E-01 101.30 3.28E-02 The tables in front of you have data on the number of gas molecules adsorbed per gram of activated carbon as a function of gas pressure. This is for two gases in two separate vessels -Methane CH4, and Ethane C2H6. Ethan Methan p/kPa n/ mol/g p/kPa n/ mol/g 7.48 3.80E-02 8.44 4.21E-03 11.38 4.61E-02 17.56 7.91E-03 19.08 5.60E-02 26.55 1.17E-02 27.79 6.70E-02 36.39 1.48E-02 37.65 7.71E-02 45.93 1.82E-02 1. According to Langmir's adsorption equation, find for each of the gases the maximum number of molecules to be adsorbed per gram of activated carbon and the adsorption constant. 2. In case the gases are both in the same vessel, write for each of the gases the equations of the dependence of the number of molecules adsorbed on the methane gas pressure and the ethane gas pressure. (Assume that the gases do not react with each other) 3. I. In which of situations 1 or 2 is the adsorption of ethane expected to be more significant than that of Methane? Explain II. Refer to the molecular structure of the gases and the results you obtained in section 1 and explain the difference in the adsorption of the gases. III. What is the type of adsorption of the gases on the surface? Explain IV. Which of the gases is more suitable for Langmir's adsorption theory? 49.98 8.54E-02 56.00 2.12E-02 61.84 9.40E-02 66.01 2.43E-02 75.19 1.02E-01 76.24 2.71E-02 90.74 1.07E-01 87.18 2.95E-02 98.33 1.08E-01 101.30 3.28E-02







