Question
The titration of 1.00 L of a 1.00 M solution of the triprotic acid, H3A, with 1.00 M NaOH is shown below. It is
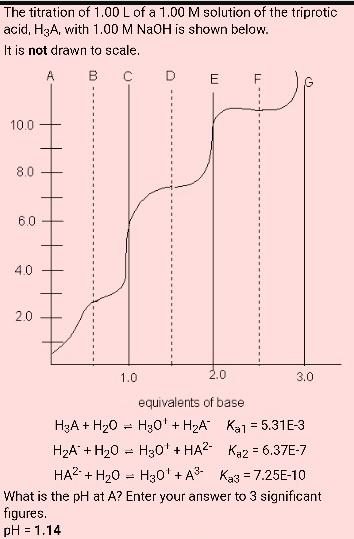
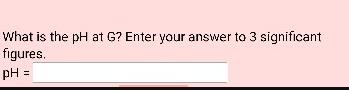
The titration of 1.00 L of a 1.00 M solution of the triprotic acid, H3A, with 1.00 M NaOH is shown below. It is not drawn to scale. A B 10.0 8.0 6.0 4.0 2.0 H3A + H0 HA + H0 1.0 = = D = E 2.0 equivalents of base H30' + HA" H30' + HA2 F 3.0 Ka1 = 5.31E-3 Kaz = 6.37E-7 HA + HO H0 + A Ka3 = 7.25E-10 What is the pH at A? Enter your answer to 3 significant figures. pH = 1.14 What is the pH at G? Enter your answer to 3 significant figures. pH =
Step by Step Solution
3.30 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
The no of moles of acid 1 L 1 molL 1 mol and the no of mol...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: L. G. Wade Jr.
8th edition
321768418, 978-0321768414
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



