Question
The titration technique is used to do the reaction because the 0.1 M NaOH can be added sequentially and the pH is monitored as the
The titration technique is used to do the reaction because the 0.1 M NaOH can be added sequentially and the pH is monitored as the moles of HA react to form NaA. The Ka is calculated at the three lettered points. Use the information is question 1 to find:
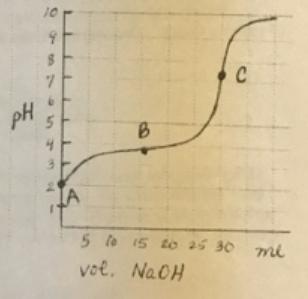
(a) How many moles of HA are present at point A (initial)?
(b) How many moles of HA are present at point B (the half-equivalence point)?
(c) How many moles of HA are present at point C (the equivalence point)?
10 pH 5 To 15 to a5 30 mi vol. NaOH 1164T3
Step by Step Solution
3.39 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Probability & Statistics For Engineers & Scientists
Authors: Ronald E. Walpole, Raymond H. Myers, Sharon L. Myers, Keying
7th Edition
9789813131279, 130415294, 9813131276, 978-0130415295
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



