Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The two Fe complexes dissolved into the solution described in Question 1(a) are then studied by Raman spectroscopy. When the Raman spectra of Complex
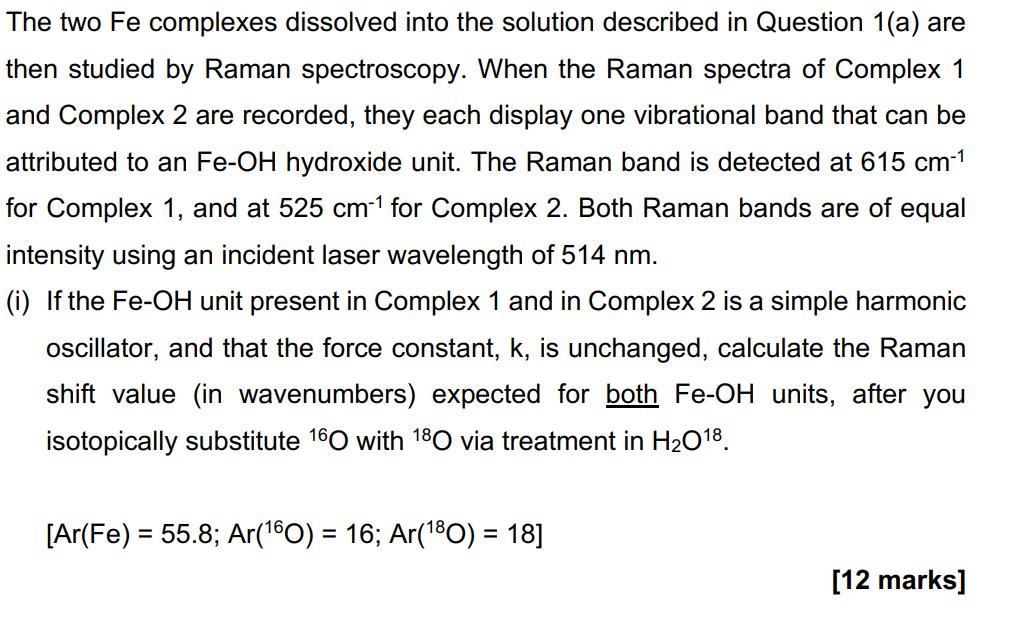
The two Fe complexes dissolved into the solution described in Question 1(a) are then studied by Raman spectroscopy. When the Raman spectra of Complex 1 and Complex 2 are recorded, they each display one vibrational band that can be attributed to an Fe-OH hydroxide unit. The Raman band is detected at 615 cm-1 for Complex 1, and at 525 cm-1 for Complex 2. Both Raman bands are of equal intensity using an incident laser wavelength of 514 nm. (i) If the Fe-OH unit present in Complex 1 and in Complex 2 is a simple harmonic oscillator, and that the force constant, k, is unchanged, calculate the Raman shift value (in wavenumbers) expected for both Fe-OH units, after you isotopically substitute 160 with 180 via treatment in H2O18. [Ar(Fe) = 55.8; Ar(160) = 16; Ar(180) = 18] [12 marks]
Step by Step Solution
★★★★★
3.48 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
To calculate the expected Raman shift value after isotopic substitution we can use t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


