Answered step by step
Verified Expert Solution
Question
1 Approved Answer
the unit below treats a mixture of tetrachloromethane ( CCl ) and chlorobenzene ( C6H5Cl) by flash distillation step by step with diagram to UNDERSTAND
the unit below treats a mixture of tetrachloromethane ( CCl ) and chlorobenzene ( C6H5Cl) by flash distillation
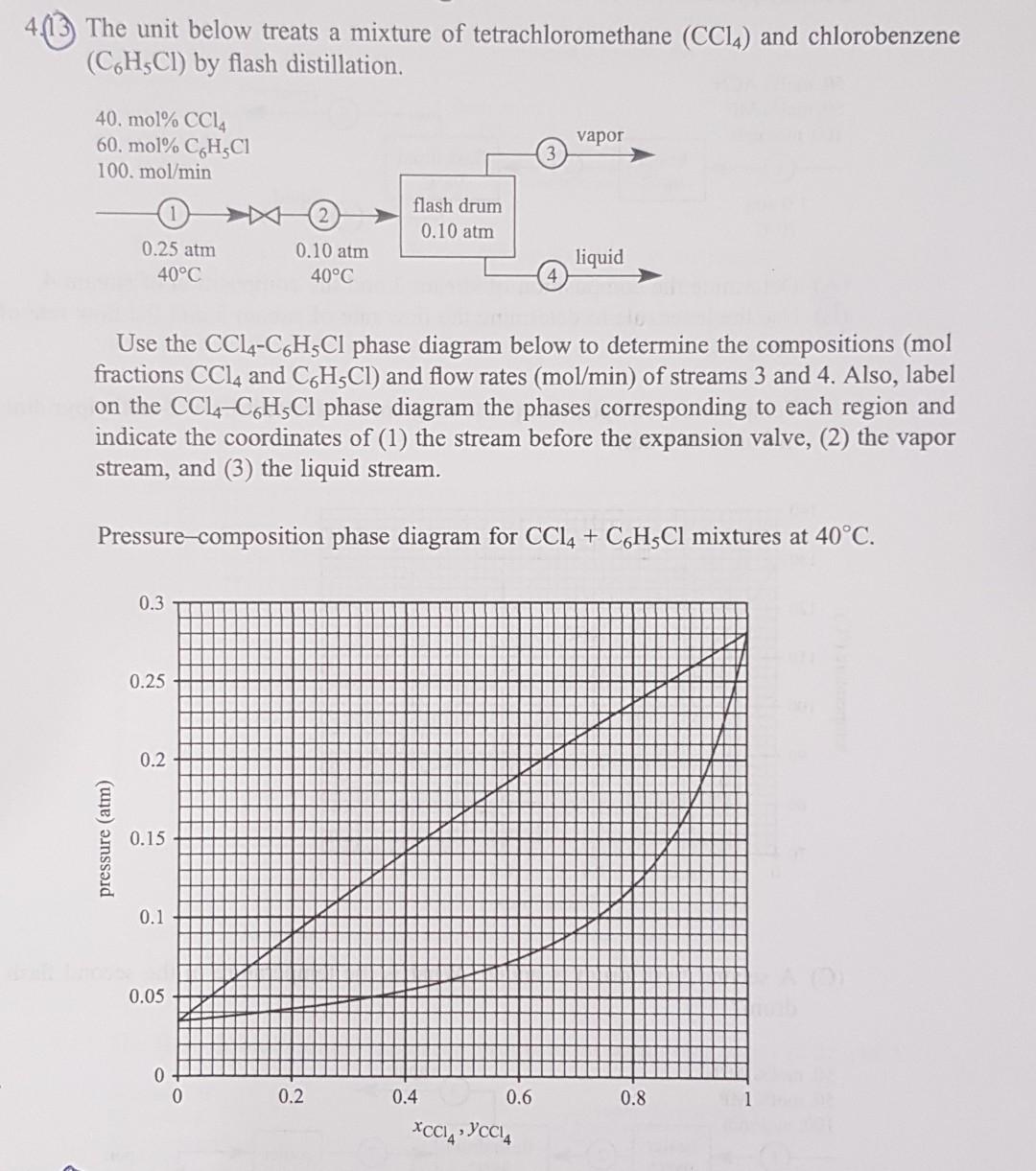
step by step with diagram to UNDERSTAND please repeat step by step please
4.(13) The unit below treats a mixture of tetrachloromethane (CCl4) and chlorobenzene (C6H5Cl) by flash distillation. Use the CCl4C6H5Cl phase diagram below to determine the compositions ( mol fractions CCl4 and C6H5Cl) and flow rates (mol/min) of streams 3 and 4 . Also, label on the CCl4C6H5Cl phase diagram the phases corresponding to each region and indicate the coordinates of (1) the stream before the expansion valve, (2) the vapor stream, and (3) the liquid stream. Pressure-composition phase diagram for CCl4+C6H5Cl mixtures at 40CStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


