Question
Xylene can be produced from toluene as written schematically: CH, CH3 CH3 AG 14,85 kJ mol CH3 toluene ortho-xylene benzene CH, CH3 CH, 10.42 kJ
Xylene can be produced from toluene as written schematically:
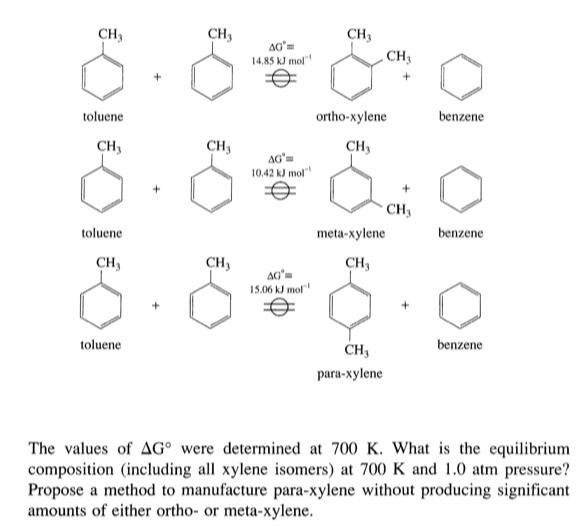

CH, CH3 CH3 AG 14,85 kJ mol CH3 toluene ortho-xylene benzene CH, CH3 CH, 10.42 kJ mol" CH3 toluene meta-xylene benzene CH3 CH, CH, 15.06 kJ mol toluene CH, benzene para-xylene The values of AG were determined at 700 K. What is the equilibrium composition (including all xylene isomers) at 700 K and 1.0 atm pressure? Propose a method to manufacture para-xylene without producing significant amounts of either ortho- or meta-xylene.
Step by Step Solution
3.40 Rating (169 Votes )
There are 3 Steps involved in it
Step: 1
The equilibrium constant for the given reaction assuming ideal gas behavior can be calculated using ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



