Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The van der Waals equation of state was designed (by Dutch physicist Johannes van der Waals) to predict the relationship between pressure p, volume
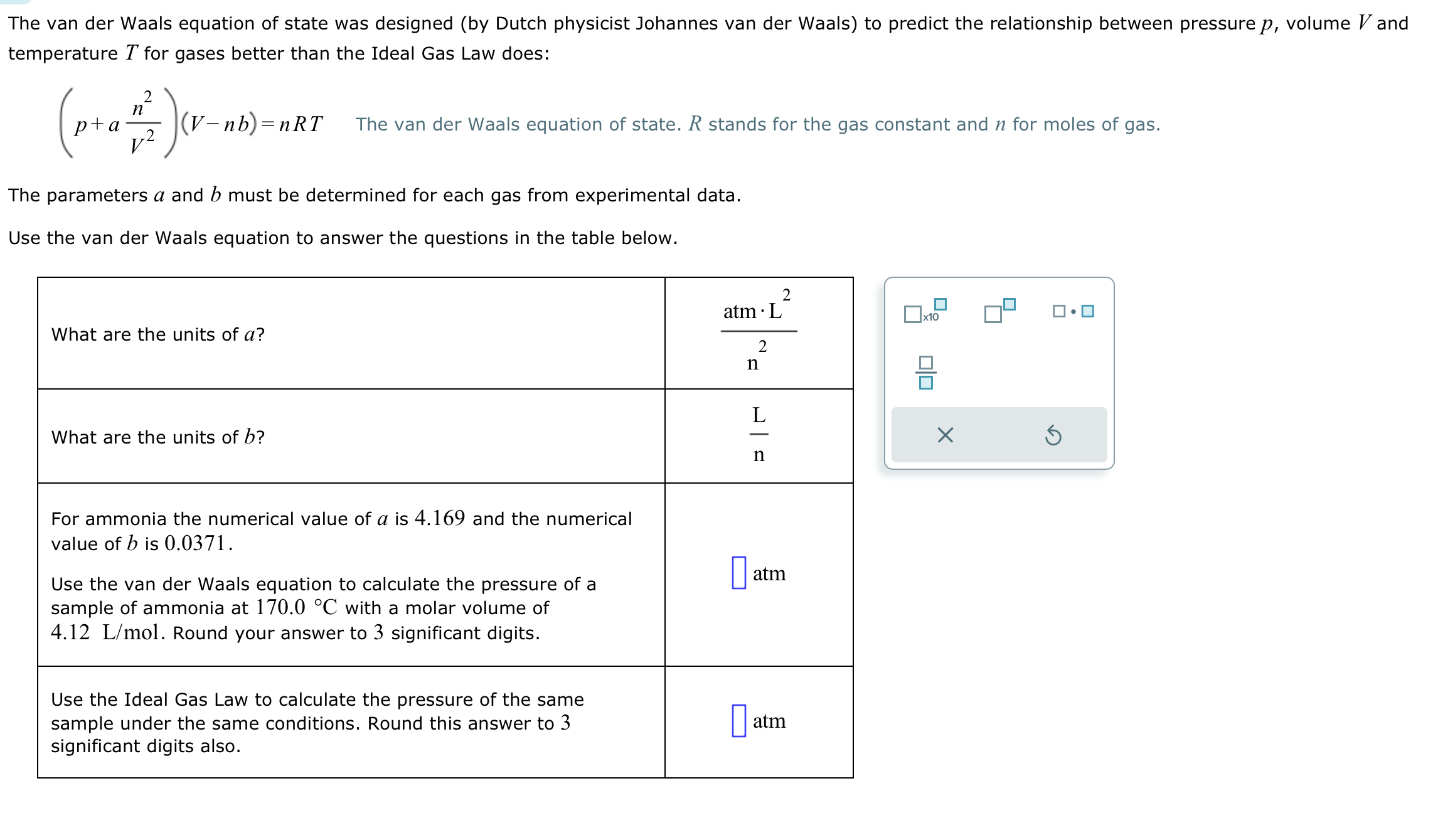
The van der Waals equation of state was designed (by Dutch physicist Johannes van der Waals) to predict the relationship between pressure p, volume Vand temperature T for gases better than the Ideal Gas Law does: 2 n (p+ = ^) ( v. (V-nb)=nRT The parameters a and b must be determined for each gas from experimental data. Use the van der Waals equation to answer the questions in the table below. What are the units of a? The van der Waals equation of state. R stands for the gas constant and n for moles of gas. What are the units of b? For ammonia the numerical value of a is 4.169 and the numerical value of b is 0.0371. Use the van der Waals equation to calculate the pressure of a sample of ammonia at 170.0 C with a molar volume of 4.12 L/mol. Round your answer to 3 significant digits. Use the Ideal Gas Law to calculate the pressure of the same sample under the same conditions. Round this answer to 3 significant digits also. atm 2 n L n 2 atm atm x10 010 X S
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The image you have provided contains the Van der Waals equation which is a modified version of the Ideal Gas Law that accounts for the volume occupied ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


