Answered step by step
Verified Expert Solution
Question
1 Approved Answer
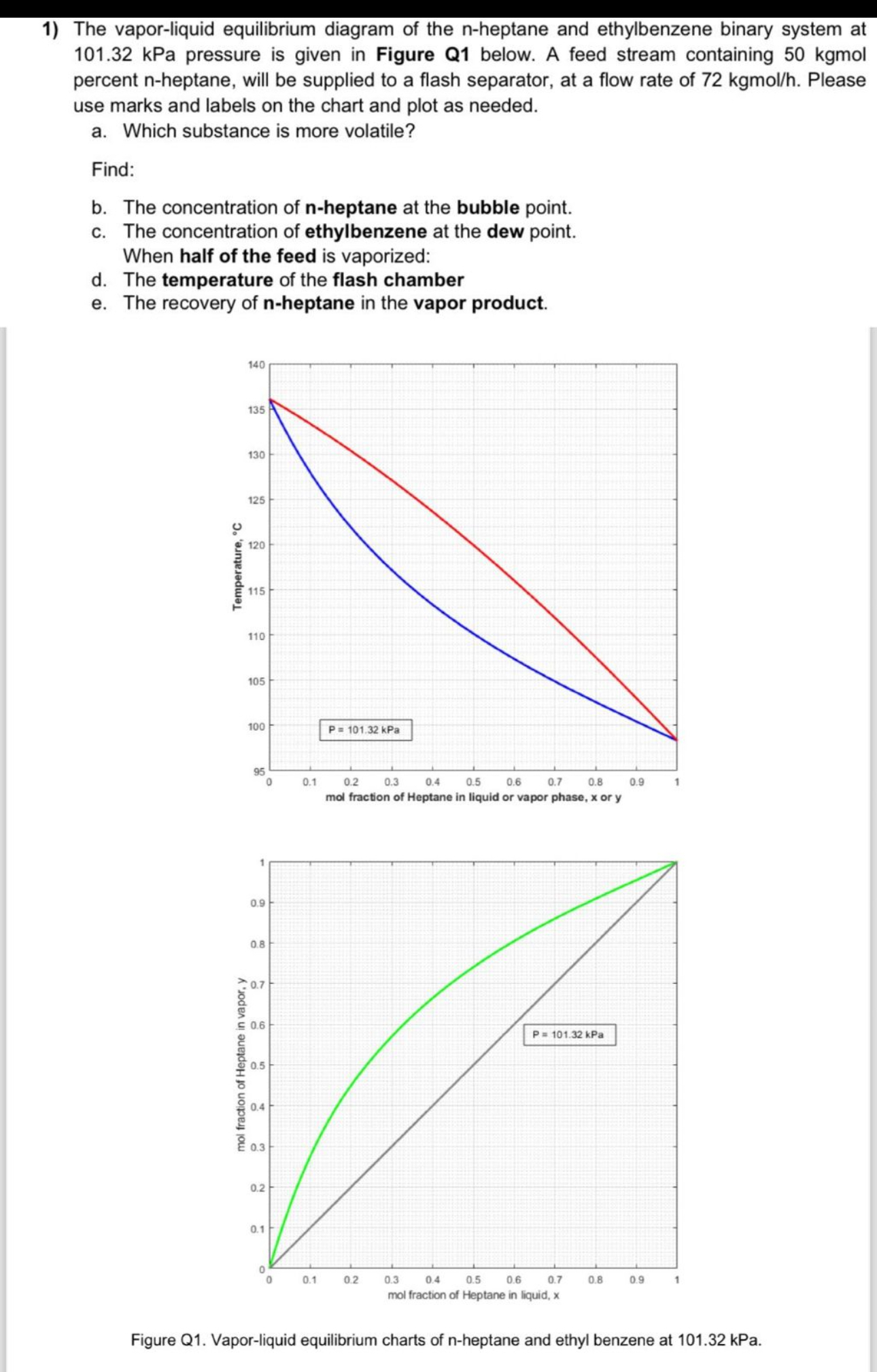
The vapor - liquid equilibrium diagram of the n - heptane and ethylbenzene binary system at 1 0 1 . 3 2 kPa pressure is
The vaporliquid equilibrium diagram of the nheptane and ethylbenzene binary system at kPa pressure is given in Figure Q below. A feed stream containing kgmol percent heptane, will be supplied to a flash separator, at a flow rate of kgmo Please use marks and labels on the chart and plot as needed.
a Which substance is more volatile?
Find:
b The concentration of heptane at the bubble point.
c The concentration of ethylbenzene at the dew point. When half of the feed is vaporized:
d The temperature of the flash chamber
e The recovery of heptane in the vapor product.
Figure Q Vaporliquid equilibrium charts of heptane and ethyl benzene at kPa.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


