Answered step by step
Verified Expert Solution
Question
1 Approved Answer
there are 2 questions for this part thank you so much in advance the second q is just answer as an epirical formula C5H10NS2,296.55g/mol Express
there are 2 questions for this part
thank you so much in advance 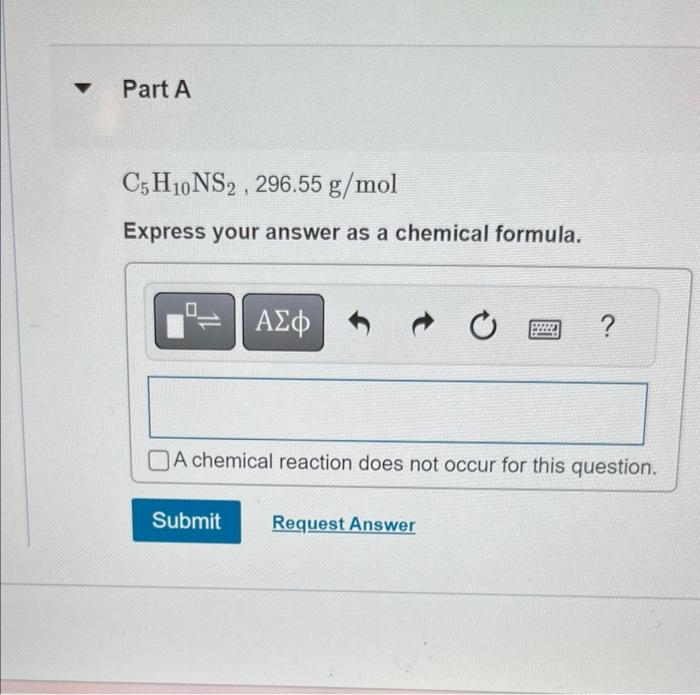


the second q is just answer as an epirical formula 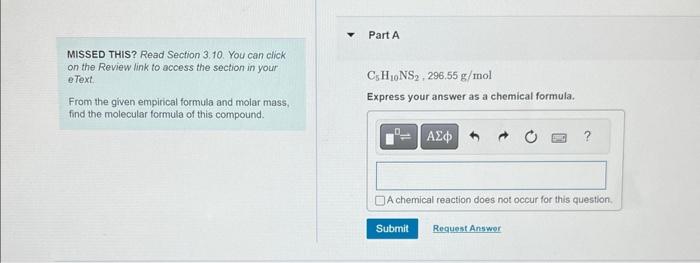

C5H10NS2,296.55g/mol Express your answer as a chemical formula. A sample of a compound is decomposed in the laboratory and produces 330g carbon, 55.6g hydrogen, and 220.2g oxygen, Calculate the empirical formula of the compound. Expross your answer as an empirical formula. MISSED THIS? Read Section 3.10. You can click on the Review link to access the section in your etext. C5H10NS2,296,55g/mol From the given empirical formula and molar mass, Express your answer as a chemical formula. find the molecular formula of this compound. A sample of a compound is decomposed in the laboratory and produces 330g carbon, 55.6g hydrogon, and 2202g orygen. Calculate the enpirical formula of the compound Express your answer as an empirical formula 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


