There are an estimated 15,000 legacy mine sites in Queensland and the following information is from one of these sites producing severe AMD. The AMD
There are an estimated 15,000 legacy mine sites in Queensland and the following information is from one of these sites producing severe AMD. The AMD is produced at a flow rate of 40 m3/h and has a sulfate concentration of 3360 mg/L.
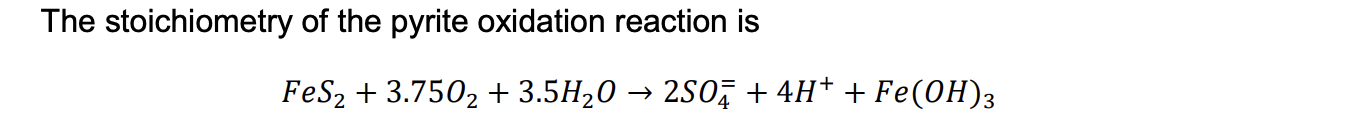
1. Determine the change in oxidation numbers of the elements in the pyrite oxidation reaction and the pH of the AMD.
2. Estimate the amount of caustic soda required in kg per year to neutralize the AMD (use the values that you need from question 1 to solve this problem).
3. For the Australian mining industry, the additional cost of managing AMD sludge at operating mine sites is estimated to be about $60 million per year. Estimate the amount of sludge generated in kg per year as Na2SO4 and the overall treatment costs considering the disposal cost of sludge (AU$100/tonne) and the cost of the caustic soda (AU$150/tonne) needed for the treatment. Assume a water content in the sludge of 40%, which must be considered in both the sludge generated per year and in the treatment costs.
4. AMD usually contains dissolved metals that are toxic and must be removed but also some that have economic value. When AMD is treated through chemical precipitation as in this example, the metals in the ionic form are converted to an insoluble form by the chemical reaction between the soluble metal compounds and the precipitating reagent. Zinc and Iron both at a concentration of 0.10 M are present in the AMD ENVE3160 Environmental Phenomena 2023 discussed in this tutorial. Zinc has a higher economic value (AU $5,000 per tonne) than iron and its recovery could offset treatment costs. The simplified metal precipitation reaction with OH (from NaOH) is M2+ + 2(OH- ) M(OH)2 Where M= is either Zn or Fe. Is it feasible to precipitate 90% of zinc but not the iron by controlling the pH (0.1)?
Iron hydroxide Fe(OH)2 Ksp: 8.0 x 10^-16
Zinc hydroxide Zn(OH)2 Ksp: 7.68 10^-17
The stoichiometry of the pyrite oxidation reaction is FeS2+3.75O2+3.5H2O2SO4=+4H++Fe(OH)3Step by Step Solution
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


