Answered step by step
Verified Expert Solution
Question
1 Approved Answer
There is a theory that methane, CH4, constantly leaks from the Earth's crust. This is not noticeable in most areas on land but at
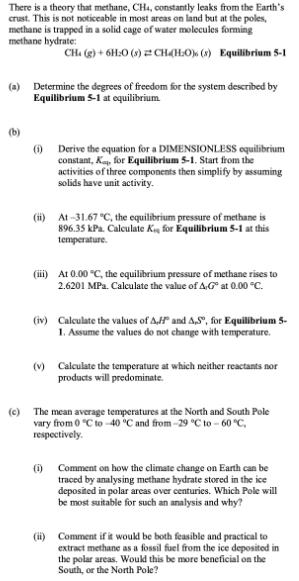
There is a theory that methane, CH4, constantly leaks from the Earth's crust. This is not noticeable in most areas on land but at the poles, methane is trapped in a solid cage of water molecules forming methane hydrate: CH. (g) + 6H:0 (s) CH (HO) (x) Equilibrium 5-1 (a) Determine the degrees of freedom for the system described by Equilibrium 5-1 at equilibrium (b) (1) Derive the equation for a DIMENSIONLESS equilibrium constant, K, for Equilibrium 5-1. Start from the activities of three components then simplify by assuming solids have unit activity. (ii) At-31.67 C, the equilibrium pressure of methane is 896.35 kPa. Calculate K, for Equilibrium 5-1 at this temperature. (iii) At 0.00 C, the equilibrium pressure of methane rises to 2.6201 MPa. Calculate the value of AG at 0.00 C. (iv) Calculate the values of AH and AS, for Equilibrium 5- 1. Assume the values do not change with temperature. (v) Calculate the temperature at which neither reactants nor products will predominate. (c) The mean average temperatures at the North and South Pole vary from 0 C to -40 C and from -29 C to -60 C, respectively. (i) Comment on how the climate change on Earth can be traced by analysing methane hydrate stored in the ice deposited in polar areas over centuries. Which Pole will be most suitable for such an analysis and why? (ii) Comment if it would be both feasible and practical to extract methane as a fossil fuel from the ice deposited in the polar areas. Would this be more beneficial on the South, or the North Pole?
Step by Step Solution
★★★★★
3.40 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Answer a Degrees of freedom f for the system at equilibrium f C 2 P where C is the number of components CH HO CH6HO 3 P is the number of phases gas an...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


