Answered step by step
Verified Expert Solution
Question
1 Approved Answer
There is an artificial pond located near a great lake. The pond is connected to the main lake through a small channel, which is used
There is an artificial pond located near a great lake. The pond is connected to the
main lake through a small channel, which is used for water intake. In winter, the pond is used
as a storage reservoir for the flow from the lake. In summer, the pond is used for irrigation
purposes. The water levels between the lake and the pond remain the same after wintertime, so
the velocity in the channel becomes zero. However, before the irrigation season, a new industry
started to discharge its wastewater into the lake. This caused the boron concentration in the lake
to rise to ppm which is hazardous if used in high concentrations for agricultural irrigation. It
is important to note that boron is an inert substance. The connecting channel is meters long,
meters wide, and meters deep. Assume the lake and the pond are completely mixed and the
diffusion constant for boron is ms
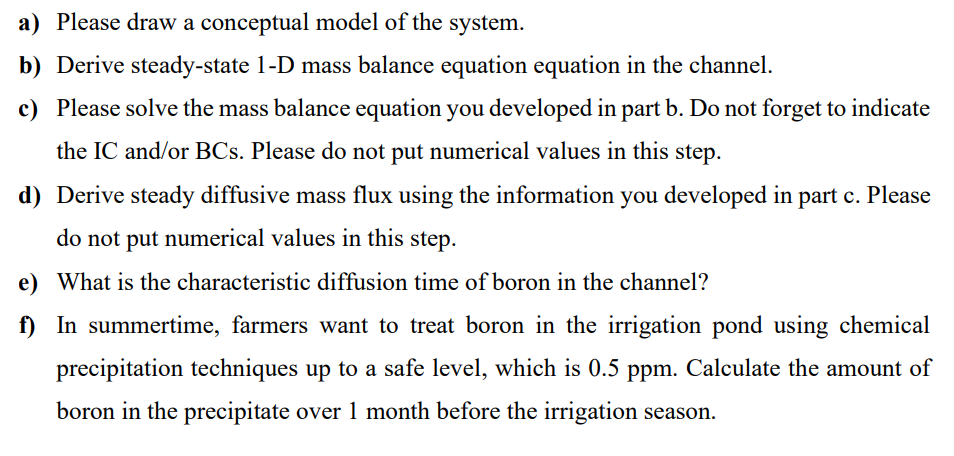
a Please draw a conceptual model of the system.
b Derive steadystate D mass balance equation equation in the channel.
c Please solve the mass balance equation you developed in part b Do not forget to indicate
the IC andor BCs Please do not put numerical values in this step.
d Derive steady diffusive mass flux using the information you developed in part c Please
do not put numerical values in this step.
e What is the characteristic diffusion time of boron in the channel?
f In summertime, farmers want to treat boron in the irrigation pond using chemical
precipitation techniques up to a safe level, which is Calculate the amount of
boron in the precipitate over month before the irrigation season.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


