Answered step by step
Verified Expert Solution
Question
1 Approved Answer
thermodynamics. pls provide full ans with legible handwriting. thanks. A propanol (1) - water (2) mixture is at equilibrium in a closed container at 60C
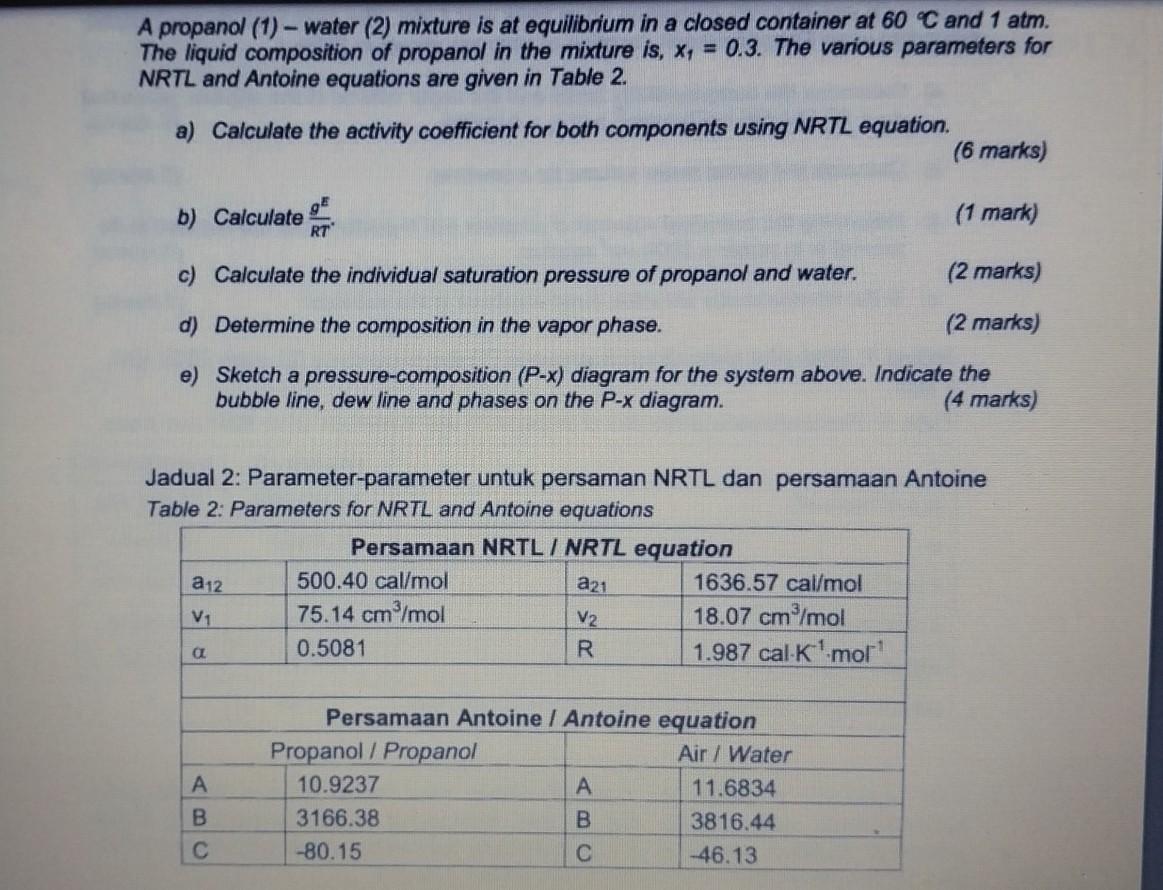
thermodynamics. pls provide full ans with legible handwriting. thanks.
A propanol (1) - water (2) mixture is at equilibrium in a closed container at 60C and 1atm. The liquid composition of propanol in the mixture is, x1=0.3. The various parameters for NRTL and Antoine equations are given in Table 2. a) Calculate the activity coefficient for both components using NRTL equation. (6 marks) b) Calculate RTgE. (1 mark) c) Calculate the individual saturation pressure of propanol and water. (2 marks) d) Determine the composition in the vapor phase. (2 marks) e) Sketch a pressure-composition (P-x) diagram for the system above. Indicate the bubble line, dew line and phases on the P-x diagram. (4 marks) Jadual 2: Parameter-parameter untuk persaman NRTL dan persamaan Antoine Table 2: Parameters for NRTL and Antoine equationsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


