Answered step by step
Verified Expert Solution
Question
1 Approved Answer
thermodynamics The excess Gibbs energy of a binary liquid mixture at T and P is given by: GE/RT=(2.6x11.8x2)x1x2 (a) Find expressions for ln1 and ln2
thermodynamics 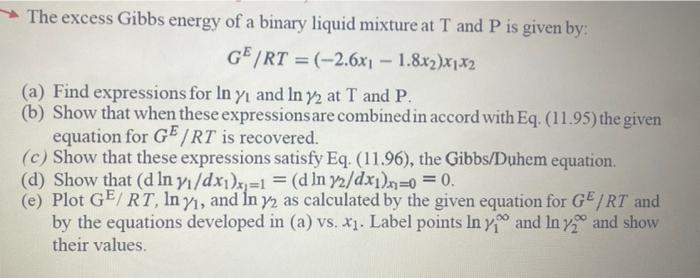
The excess Gibbs energy of a binary liquid mixture at T and P is given by: GE/RT=(2.6x11.8x2)x1x2 (a) Find expressions for ln1 and ln2 at T and P. (b) Show that when these expressionsare combined in accord with Eq. (11.95) the given equation for GE/RT is recovered. (c) Show that these expressions satisfy Eq. (11.96), the Gibbs/Duhem equation. (d) Show that (dln1/dx1)x1=1=(dln2/dx1)x1=0=0. (e) Plot GE/RT,ln1, and ln2 as calculated by the given equation for GE/RT and by the equations developed in (a) vs. x1. Label points ln1 and ln2 and show their values 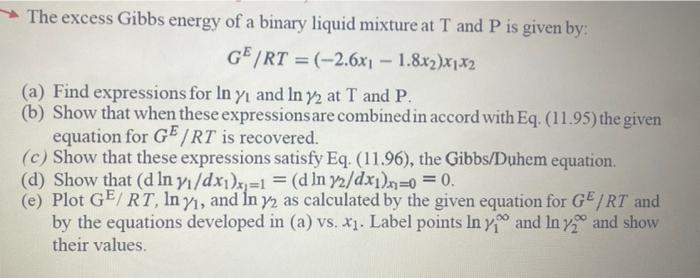
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


