Answered step by step
Verified Expert Solution
Question
1 Approved Answer
These are information please calculate the theoretical yield and the other boxes Background Information The following chapters from your textbook will provide valuable background information:

These are information please calculate the theoretical yield and the other boxes
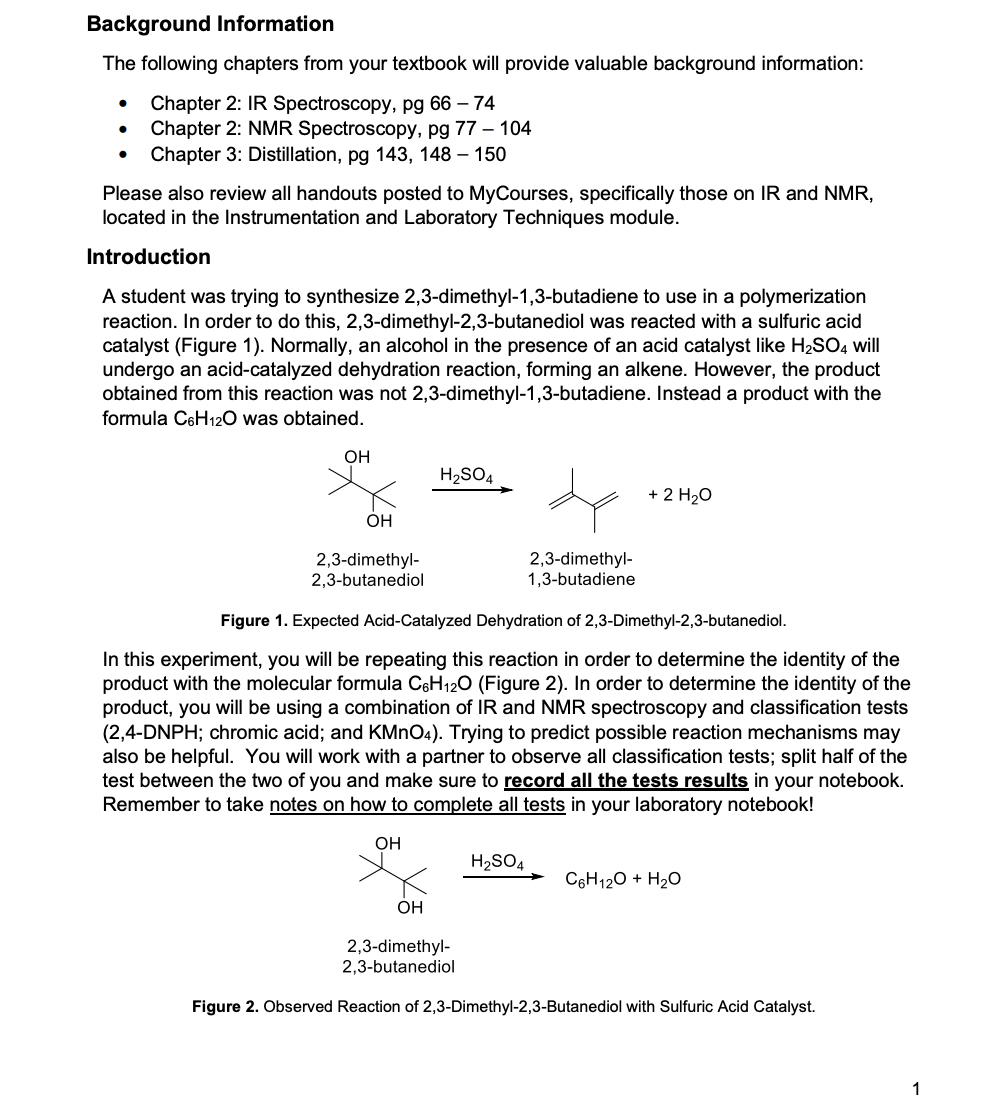
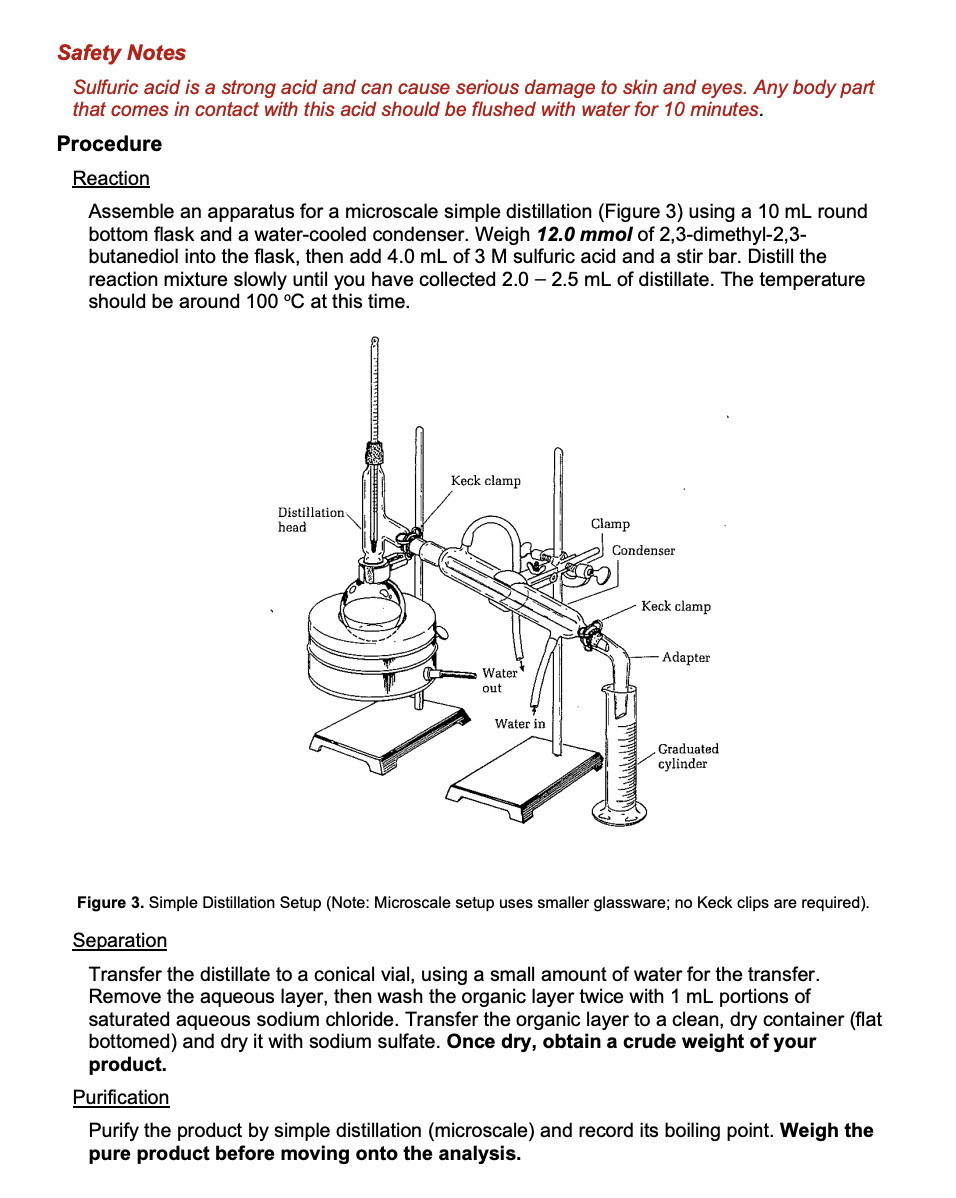
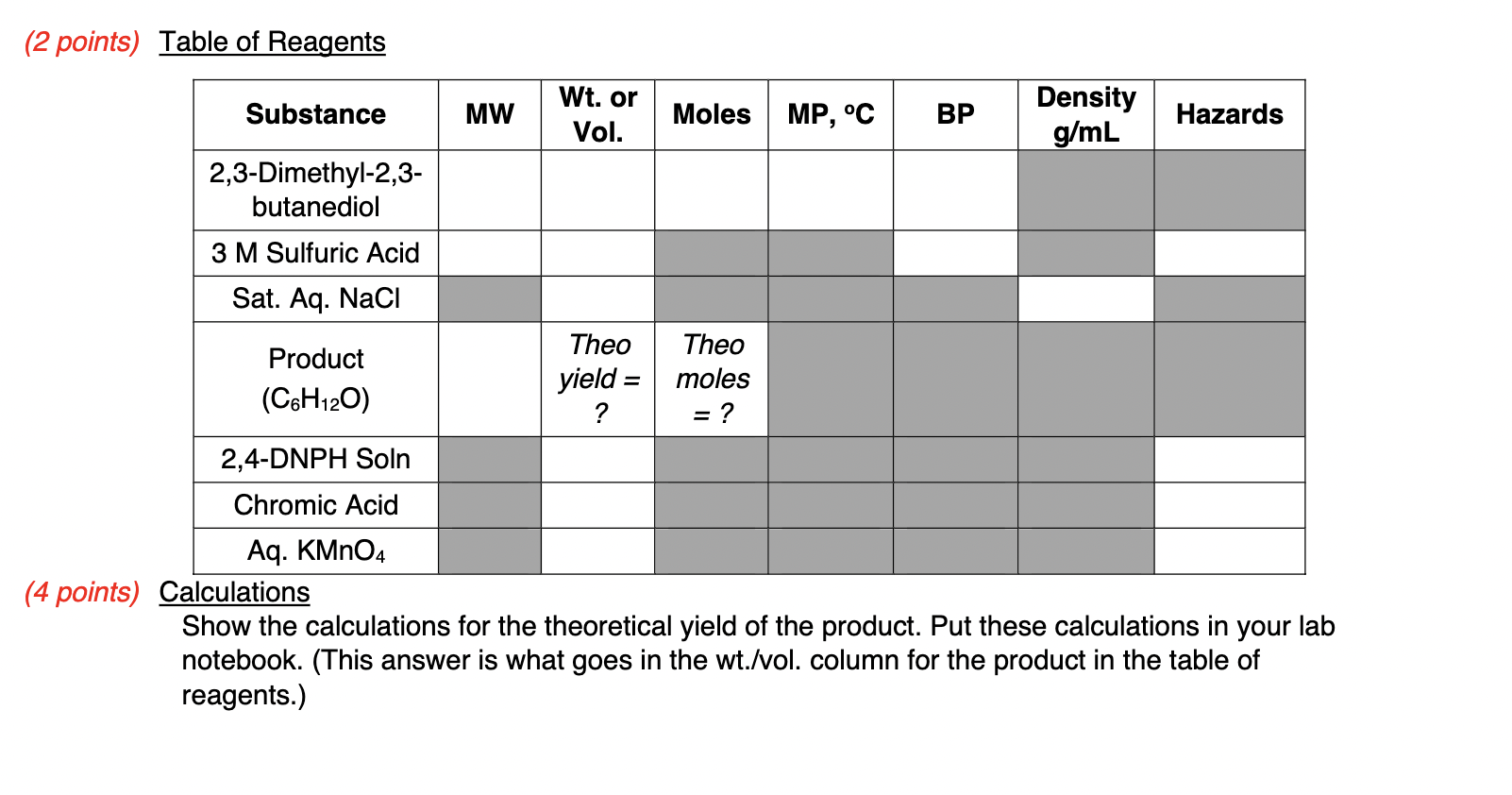
Background Information The following chapters from your textbook will provide valuable background information: - Chapter 2: IR Spectroscopy, pg 6674 - Chapter 2: NMR Spectroscopy, pg 77104 - Chapter 3: Distillation, pg 143, 148 - 150 Please also review all handouts posted to MyCourses, specifically those on IR and NMR, located in the Instrumentation and Laboratory Techniques module. Introduction A student was trying to synthesize 2,3-dimethyl-1,3-butadiene to use in a polymerization reaction. In order to do this, 2,3-dimethyl-2,3-butanediol was reacted with a sulfuric acid catalyst (Figure 1). Normally, an alcohol in the presence of an acid catalyst like H2SO4 will undergo an acid-catalyzed dehydration reaction, forming an alkene. However, the product obtained from this reaction was not 2,3-dimethyl-1,3-butadiene. Instead a product with the formula C6H12O was obtained. Figure 1. Expected Acid-Catalyzed Dehydration of 2,3-Dimethyl-2,3-butanediol. In this experiment, you will be repeating this reaction in order to determine the identity of the product with the molecular formula C6H12O (Figure 2). In order to determine the identity of the product, you will be using a combination of IR and NMR spectroscopy and classification tests (2,4-DNPH; chromic acid; and KMnO4 ). Trying to predict possible reaction mechanisms may also be helpful. You will work with a partner to observe all classification tests; split half of the test between the two of you and make sure to record all the tests results in your notebook. Remember to take notes on how to complete all tests in your laboratory notebook! H2SO4C6H12O+H2O 2,3-dimethyl- 2,3-butanediol Figure 2. Observed Reaction of 2,3-Dimethyl-2,3-Butanediol with Sulfuric Acid Catalyst. Safety Notes Sulfuric acid is a strong acid and can cause serious damage to skin and eyes. Any body part that comes in contact with this acid should be flushed with water for 10 minutes. Procedure Reaction Assemble an apparatus for a microscale simple distillation (Figure 3) using a 10mL round bottom flask and a water-cooled condenser. Weigh 12.0mmol of 2,3-dimethyl-2,3butanediol into the flask, then add 4.0mL of 3M sulfuric acid and a stir bar. Distill the reaction mixture slowly until you have collected 2.02.5mL of distillate. The temperature should be around 100C at this time. Figure 3. Simple Distillation Setup (Note: Microscale setup uses smaller glassware; no Keck clips are required). Separation Transfer the distillate to a conical vial, using a small amount of water for the transfer. Remove the aqueous layer, then wash the organic layer twice with 1mL portions of saturated aqueous sodium chloride. Transfer the organic layer to a clean, dry container (flat bottomed) and dry it with sodium sulfate. Once dry, obtain a crude weight of your product. Purification Purify the product by simple distillation (microscale) and record its boiling point. Weigh the pure product before moving onto the analysis. You can perform the following in any order. All classification tests should also be completed with a known positive to compare results. Chromic Acid Test Dissolve 1 drop of the product in 1.0mL of reagent grade acetone in a test tube. Add 1 drop of the chromic acid reagent and swirl, noting the time required for a positive test. The formation of an opaque blue-green suspension within 23 seconds, accompanied by disappearance of the orange color of the reagent, indicates a primary or secondary alcohol. Aldehydes give the same result but react more slowly, requiring 3090 seconds to react. The generation of some other dark color, particularly with the color of the liquid remaining orange, should be considered a negative test. 2,4-DNPH Test Dissolve 1 drop of the product in 1.0mL of 95% ethanol; use more ethanol if necessary. Add this solution to 2.0mL of the DNPH reagent in a test tube. Stopper and shake the test tube and let the mixture stand for 15 minutes or until a precipitate forms. If no precipitate has formed after 15 minutes, scratch the inside of the test tube with a glass stirring rod. The formation of a yellow/orange precipitate indicates an aldehyde or ketone. Potassium Permanganate Dissolve 1 drop of the product in 2.0mL of water or 95% ethanol (if the product is insoluble in water). Add 0.10M potassium permanganate drop by drop until the purple color of the permanganate persists or until about 20 drops have been added. If a reaction doesn't take place immediately, shake the mixture and let it stand for up to 5 minutes. Disregard any decolorization that takes place after 5 minutes. Decolorization of more than one drop of the purple permanganate solution, accompanied by the formation of a brown precipitate of manganese dioxide, suggests carbon-carbon unsaturation. IR Spectroscopy Obtain an IR, 1H, and 13C NMR spectra from your TA to be analyzed and uploaded with your report. The IR spectrum of the starting material can be found on MyCourses. This will be helpful in comparison for your report. (2 points) Table of Reagents (4 points) Calculations Show the calculations for the theoretical yield of the product. Put these calculations in your lab notebook. (This answer is what goes in the wt./vol. column for the product in the table of reagents.) Background Information The following chapters from your textbook will provide valuable background information: - Chapter 2: IR Spectroscopy, pg 6674 - Chapter 2: NMR Spectroscopy, pg 77104 - Chapter 3: Distillation, pg 143, 148 - 150 Please also review all handouts posted to MyCourses, specifically those on IR and NMR, located in the Instrumentation and Laboratory Techniques module. Introduction A student was trying to synthesize 2,3-dimethyl-1,3-butadiene to use in a polymerization reaction. In order to do this, 2,3-dimethyl-2,3-butanediol was reacted with a sulfuric acid catalyst (Figure 1). Normally, an alcohol in the presence of an acid catalyst like H2SO4 will undergo an acid-catalyzed dehydration reaction, forming an alkene. However, the product obtained from this reaction was not 2,3-dimethyl-1,3-butadiene. Instead a product with the formula C6H12O was obtained. Figure 1. Expected Acid-Catalyzed Dehydration of 2,3-Dimethyl-2,3-butanediol. In this experiment, you will be repeating this reaction in order to determine the identity of the product with the molecular formula C6H12O (Figure 2). In order to determine the identity of the product, you will be using a combination of IR and NMR spectroscopy and classification tests (2,4-DNPH; chromic acid; and KMnO4 ). Trying to predict possible reaction mechanisms may also be helpful. You will work with a partner to observe all classification tests; split half of the test between the two of you and make sure to record all the tests results in your notebook. Remember to take notes on how to complete all tests in your laboratory notebook! H2SO4C6H12O+H2O 2,3-dimethyl- 2,3-butanediol Figure 2. Observed Reaction of 2,3-Dimethyl-2,3-Butanediol with Sulfuric Acid Catalyst. Safety Notes Sulfuric acid is a strong acid and can cause serious damage to skin and eyes. Any body part that comes in contact with this acid should be flushed with water for 10 minutes. Procedure Reaction Assemble an apparatus for a microscale simple distillation (Figure 3) using a 10mL round bottom flask and a water-cooled condenser. Weigh 12.0mmol of 2,3-dimethyl-2,3butanediol into the flask, then add 4.0mL of 3M sulfuric acid and a stir bar. Distill the reaction mixture slowly until you have collected 2.02.5mL of distillate. The temperature should be around 100C at this time. Figure 3. Simple Distillation Setup (Note: Microscale setup uses smaller glassware; no Keck clips are required). Separation Transfer the distillate to a conical vial, using a small amount of water for the transfer. Remove the aqueous layer, then wash the organic layer twice with 1mL portions of saturated aqueous sodium chloride. Transfer the organic layer to a clean, dry container (flat bottomed) and dry it with sodium sulfate. Once dry, obtain a crude weight of your product. Purification Purify the product by simple distillation (microscale) and record its boiling point. Weigh the pure product before moving onto the analysis. You can perform the following in any order. All classification tests should also be completed with a known positive to compare results. Chromic Acid Test Dissolve 1 drop of the product in 1.0mL of reagent grade acetone in a test tube. Add 1 drop of the chromic acid reagent and swirl, noting the time required for a positive test. The formation of an opaque blue-green suspension within 23 seconds, accompanied by disappearance of the orange color of the reagent, indicates a primary or secondary alcohol. Aldehydes give the same result but react more slowly, requiring 3090 seconds to react. The generation of some other dark color, particularly with the color of the liquid remaining orange, should be considered a negative test. 2,4-DNPH Test Dissolve 1 drop of the product in 1.0mL of 95% ethanol; use more ethanol if necessary. Add this solution to 2.0mL of the DNPH reagent in a test tube. Stopper and shake the test tube and let the mixture stand for 15 minutes or until a precipitate forms. If no precipitate has formed after 15 minutes, scratch the inside of the test tube with a glass stirring rod. The formation of a yellow/orange precipitate indicates an aldehyde or ketone. Potassium Permanganate Dissolve 1 drop of the product in 2.0mL of water or 95% ethanol (if the product is insoluble in water). Add 0.10M potassium permanganate drop by drop until the purple color of the permanganate persists or until about 20 drops have been added. If a reaction doesn't take place immediately, shake the mixture and let it stand for up to 5 minutes. Disregard any decolorization that takes place after 5 minutes. Decolorization of more than one drop of the purple permanganate solution, accompanied by the formation of a brown precipitate of manganese dioxide, suggests carbon-carbon unsaturation. IR Spectroscopy Obtain an IR, 1H, and 13C NMR spectra from your TA to be analyzed and uploaded with your report. The IR spectrum of the starting material can be found on MyCourses. This will be helpful in comparison for your report. (2 points) Table of Reagents (4 points) Calculations Show the calculations for the theoretical yield of the product. Put these calculations in your lab notebook. (This answer is what goes in the wt./vol. column for the product in the table of reagents.)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


