Answered step by step
Verified Expert Solution
Question
1 Approved Answer
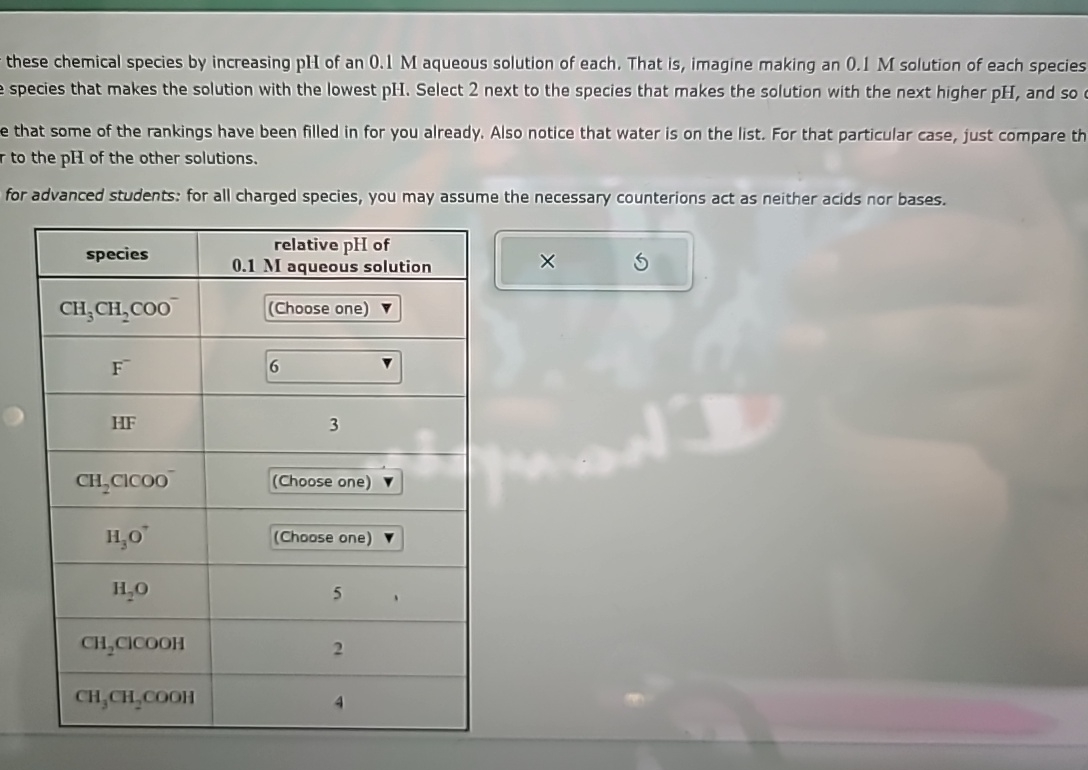
these chemical species by increasing p H of an 0 . 1 M aqueous solution of each. That is , imagine making an 0 .
these chemical species by increasing of an aqueous solution of each. That is imagine making an solution of each species species that makes the solution with the lowest Select next to the species that makes the solution with the next higher and so e that some of the rankings have been filled in for you already. Also notice that water is on the list. For that particular case, just compare th ro the of the other solutions.
for advanced students: for all charged species, you may assume the necessary counterions act as neither acids nor bases.
tablespeciestablerelative of aqueous solutionChoose one
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


