Answered step by step
Verified Expert Solution
Question
1 Approved Answer
This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto' means 10 any SI
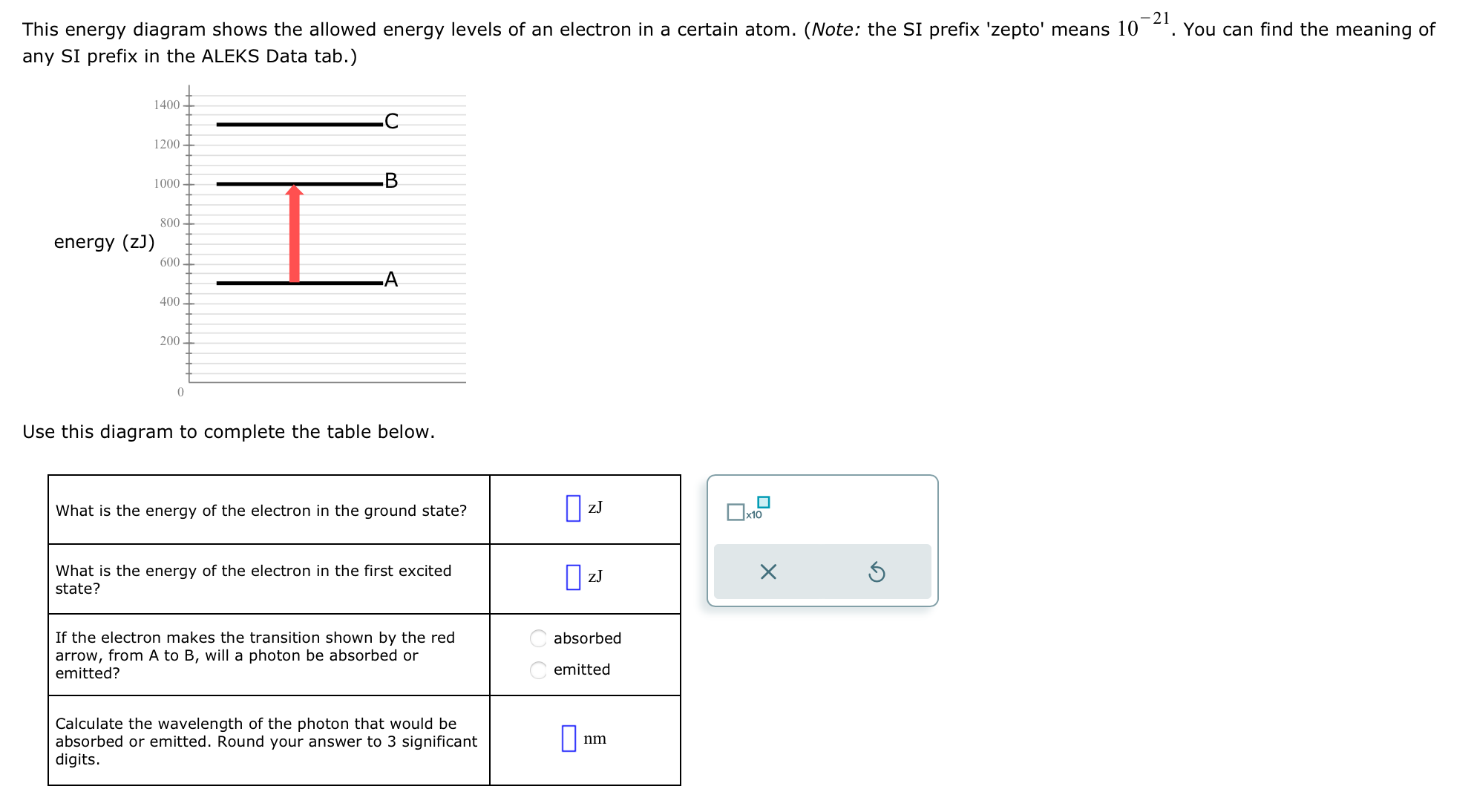
This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto' means 10 any SI prefix in the ALEKS Data tab.) 1400 1200 .C 1000 B energy (zJ) 800 600 400 200 0 Use this diagram to complete the table below. What is the energy of the electron in the ground state? zJ x10 What is the energy of the electron in the first excited state? ZJ If the electron makes the transition shown by the red arrow, from A to B, will a photon be absorbed or emitted? absorbed emitted Calculate the wavelength of the photon that would be absorbed or emitted. Round your answer to 3 significant digits. nm -21 You can find the meaning of
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


