Question: This experiment is based on parallel combinatorial esterification. Please give me a hand and explain how to solve these prelab questions. I am totally lost
This experiment is based on parallel combinatorial esterification. Please give me a hand and explain how to solve these prelab questions. I am totally lost and don't know how to solve them. Thank you!!

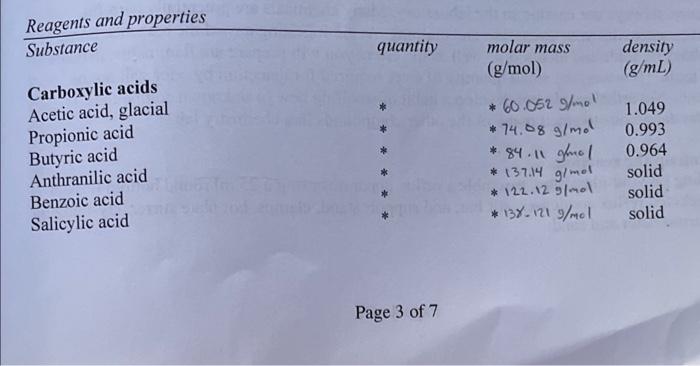


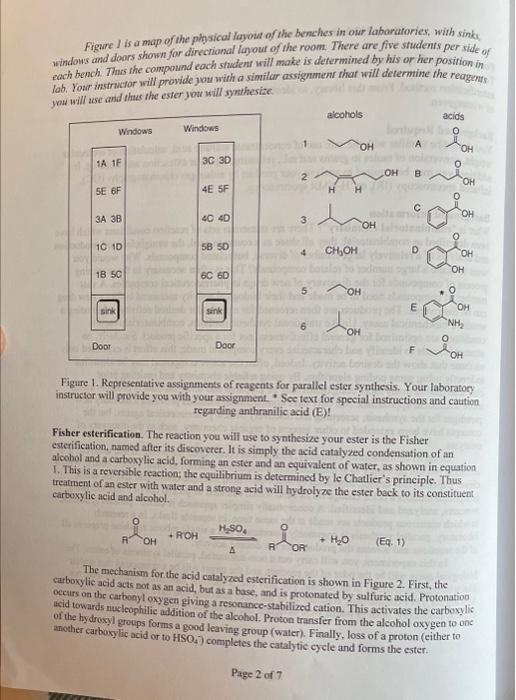
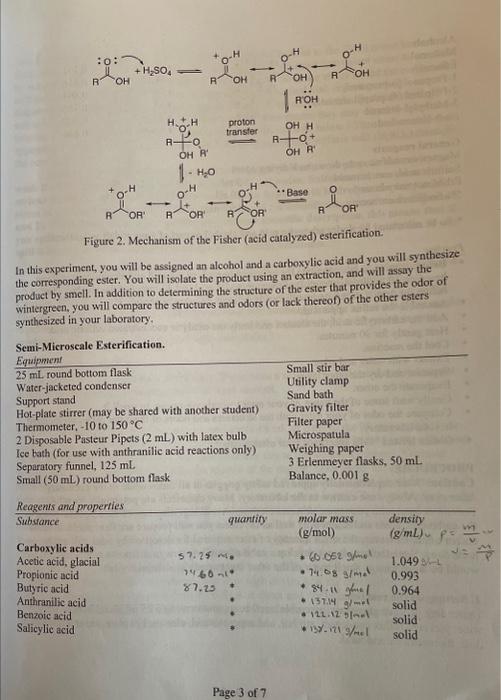



Post-laboratory questions. 1. Calculate your percent yield. 2. Discuss whether your IR and NMR spectra provide evidence for the formation of an ester and whether there is unreacted starting material still present. 3. Results table of ester name and structure versus what it smells like. \begin{tabular}{l|l} Ester Name & Ester Structure \\ \hline \end{tabular} 4. Do you notice any patterns or relationships between the chemical structures and the odors? Describe them. Pre-laboratory assignment. 1. What safety precautions should be taken when using concentrated sulfuric acid? 2. Calculate the grams (for solids) or the mL (for liqu to number of mmol for your alcohol and carboxy apropriate You will need to calculate the molecular weights of your reagents. 3. Draw the structure of the ester you will synthesize. 4. Calculate the theoretical yield (in grams) of your product. Show your calculations here and in your notebook. Hint: You will need to calculate the molecular weight of your product ester. Page 6 of 7 Page 3 of 7 * To be calculated for your carboxylic acid and alcohol as part of your prelab assignment. Chem 212 Lab: Parallel Combinatorial Esterification Purpose of the Experiment To prepare a library of esters from six carboxylic acids and six alcohols using the Fisher esterification method. From this library, to identify the compound that is responsible for the odor of wintergreen. Background Required You should be familiar with the safe use of concentrated sulfuric acid, calculation of molecular weight, conversion of moles to grams and mL, refluxing, aqueous extraction and work-up. In addition, you may be required to analyze your product by cither gas chromatography (GC). infrared spectroscopy (IR), or H-NMRspectroscopy. Background Information What is "combinatorial chemistry"? There are numerous sets of reaction conditions which have been described as combinatorial; they all share as a common goal, the straightforward production and analysis of a relatively large number of diverse, but related compounds. Why would you want to make a large number of related compounds? This is often necessary in the screening process of compounds for the discovery of new drugs in pharmaceutical companies. A vast number of compounds need to be made and their biological activity measured before a new drug can be discovered. The products are then screened for a particular desired activity, often using a sensitive biochemical assay. Unlike traditional synthetic methods, combinatorial reaction conditions are optimized for general effectiveness, not for a specific product. Similarly, no attempt is made to identify and characterize every compound made; this effort is expended only on those that show the desired activity. Indeed, often the active compound may be identified indirectly using some coding algorithm. By using reliable chemistry and omitting prior characterization many more compounds may be prepared and screened than is possible using more traditional approaches. Not only have combinatorial methods provided a radically new paradigm for the synthesis, screening and discovery of new pharmaceutical agents, but the concepts have been extended to other aspects of organic chemistry, such as the discovery of catalysts. Often in combinatorial chemistry, mixtures of compounds are prepared and assayed; it is common but not necessary to synthesize these compounds attached to small plastic beads. With a clever experimental design, thousands of beads each hold a unique compound and the active compound induces a color change on its bead which can then be picked out of a mixture using tweezers, analyzed and identified. A conceptually simpler approach is called "parallel synthesis," in which numerous products are made at the same time, in separate flasks. In a research laboratory, parallel synthesis is commonly automated, and a single robotic machine prepares all of the reactions and screens the products for the desired activity. In this experiment, you will carry out a non-automated, parallel synthesis of esters. The biological assay will be the odor of the product, and the target will be a compound that smells like wintergreen. (And as in all science, you may find something interesting that you were not looking for!) For comparison, consider all of the other reactions you have carried out in the organic laboratory, most of which were designed and optimized to make a single product. Figure I is a map of the physical layout of the benches in our laboratories, with sinks windows and doors shown for directional layout of the room. There are five students per side of each bench. Thus she compound cach shdent will make is determined by his or her position in. lab. Your instructor sill provide you with a similar assignment that will determine the reagents you will use and thas the ester you will syntheriec. Figure 1. Representative assignments of reagents for parallel ester synthesis. Your laboratocy instructor will provide you with your asxignment. " See text for special instructions and caution. regarding anthranilic acid (E)! Fisher esterification. The reaction you will use to synthesize your ester is the Fisher esterification, named after its discoverer. It is simply the acid catalyzed condensation of an alcohol and a carboxylic acid, forming an ester and an equivalent of water, as shown in equation 1. This is a reversible reaction; the equilibrium is determined by le Chatlier's principle. Thus treatment of ap ester with water and a strong acid will hydrolyze the ester back to its constituent carboxylic acid and alcohol. (Eq.1) The mechaniom for the acid catalyzed esterification is shown in Figure 2. First, the curboxylic acid acts not as an acid, but as a base, and is protonated by sulfuric acid. Protonation occurs on the carbonyl oxygen giving a resonance-stabilized cation. This activates the carboxylic acid towards nucleophilic addition of the alcobol. Proton transfer from the alcohol oxygen to onc of the hydroxyl grocps forms a good leaving group (water). Finally, loss of a proton (cither toanother carboxylic acid or to HSO4 ) completes the catalytic cycle and forms the ester. Figure 2. Mechanism of the Fisher (acid catalyzed) esteruncanon In this experiment, you will be assigned an alcohol and a carboxylic acid and you will synthesize the corresponding ester. You will isolate the product using an extraction, and will assay the product by smell. In addition to determining the structure of the ester that provides the odor of wintergreen, you will compare the structures and odors (or lack thereof) of the other esters synthesized in your laboratory, - To be calculated for your carboxylic acid and alcohol as part of your prelab assignment. Preview - Before coming to lab, calculate the grams and/or mL of the acid and alcohol you will use, based on 10 millimoles (mmol) of the carboxylic acid and 40mmol of the alcohol. (Sec below for details. Some carboxylic acids will use different quantities.) - Assemble the reflux apparatus. - Add the carboxylic acid, the alcohol and lastly the catalytic concentrated sulfuric acid. - Heat gently for 30 minutes. - Cool the reaction. - Extract with water and ether. - Separate and dry the ether layer. - Evaporate the ether. - Gently waft some of the vapor of your ester towards your nose, to smell it. - Compare the odor of your ester with the structures and odors of the other esters synthesized in your laboratory. - Obtain IR and NMR spectra of your ester product. PROCEDURE Caution: Wear departmentally approved safety goggles at all times while in the chemistry laboratory. Always use caution in the laboratory. Many chemicals are potentially harmful. Prevent contact with your eyes, skin and clothing. Avoid ingesting any of the reagents. Some of the carboxylic acids and alcohols as well as the esters you will prepare are flammable, irritants. andlor toxic. However, the small quantity of ester required for detection by odor will not be hazardous. Nevertheless, do not develop a habit of smelling new or unknown compounds. It would be dangerous to smell many of the other compounds in a chemistry lab, 1. Setting up the reaction. Depending on your glasswure, assemble a reflux apparatus using a 25ml round bottom flask, a Water jacketed reflux condenser, stir bar, and support stand, clamps, hot plate stirrer, crystallizing dish, thermometer and sand. 2. Refluxing the reaction. Reflux gently for approximately 30 minutes. If you have a solid carboxylic acid and it does not dissolve completely upon heating, add a bit more alcohol. 3. Working up the reaction. To work up the reaction, cool the mixture, add about 15mL of ether to the 25ml round bottom flask and transfer the liquid to a small separatory funnel. Wash the round bottom flask with about 10mL of water and 10mL of ether and again transfer this to the separatory funnel. You may wish to add a little more ether to make the volume of the organic layer easier to handle. Extract the ether three or four times with 15 to 20mL portions of 5% aqueous sodium bicarbonate and then once with distilled water. (Will ether be the top or the bottom layer? Check with your instructor if you are not certain.) Unreacted acid will extract into the aqueous base, and all the alcohols are water soluble. Separate the ether layer and dry it with magnesium sulfate (MgSO4). Remove the MgSO4 by filtration through a fluted filter paper sitting a filling funnel; collect the ether layer in a tared round bottom flask. Carefully remove the ether using the rotatory evaporator; the lower molecular weight esters will have relatively low boiling points and will evaporate completely if the vacuum pressure is to high or you heat the distillation pot to high. Make sure the rotatory evaporator collection flask is clean and dry in case you accidentally distill your ester. A general rule: do not dispose of any of your layers until you have finished the workup and are certain that you have isolated your product. 4. Characterization of the products; determination of the odors. Weigh the round bottom flask that now contains your ester product. This will enable you to calculate the percent yield of your reaction. Carefully and gently waft some of the ester vapor towards your nose and sniff. Only a very small quantity is necessary to detect the odor. Record a description of the odor of your ester. Label your round bottom flask using a non-permanent black marker with the structure of your ester and leave it in the hood so that others can smell the ester that you synthesized. You should smell all of the other esters produced in your laboratory. Which one has the odor of wintergreen? Can you recognize any other odors? Record the chemical structures of the other esters and the odors, or lack of odor. 5. Infrared spectrum of your product. Prepare a sample of your ester product for infrared (IR) analysis. Obtain the IR spectrum as directed by your laboratory instructor. 6. Nuclear Magnetic Resonance (NMR) spectrum of your product. Prepare a sample of your ester for NMR analysis. This will generally require dissolving a drop of your ester in approximately 0.75mL of 99.8%CDCl3 and transferring this solution into an NMR tube. Obtain an NMR spectrum. 7. Cleaning up. Use the labeled collection containers provided by your laboratory instructor for disposal of waste chemicals. Be extremely careful when cleaning the reaction vessel. Wearing gloves, you should first carefully rinse it under cold water to remove all traces of sulfuric acid; even a small amount of residual concentrated sulfuric acid can burn you. Clean your glassware with soap or detergent before leaving the laboratory. Post-laboratory questions. 1. Calculate your percent yield. 2. Discuss whether your IR and NMR spectra provide evidence for the formation of an ester and whether there is unreacted starting material still present. 4. Do you notice any patterns or relationships between the chemical structures anu Describe them. Pre-laboratory assignment. 1. What safety precautions should be taken when using concentrated sulfuric acid? 1. What paretection yecagles, gloves, do nat inhale it 2. Calculate the grams (for solids) or the mL (for liquids) required to give the appropriate number of mmol for your alcohol and carboxylic acid. Hint: The density of the liquids is given. You will need to calculate the molecular weights of your reagents. 3. Draw the structure of the ester you will synthesize, 4. Calculate the theoretical yield (in grams) of your product. Show your calculations here and in your notebook. Hint: You will need to calculate the molecular weight of your product ester
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


