This figure (Figure 1)shows a container that is sealed at the top by a movable piston. Inside the container is an gas at 1.00
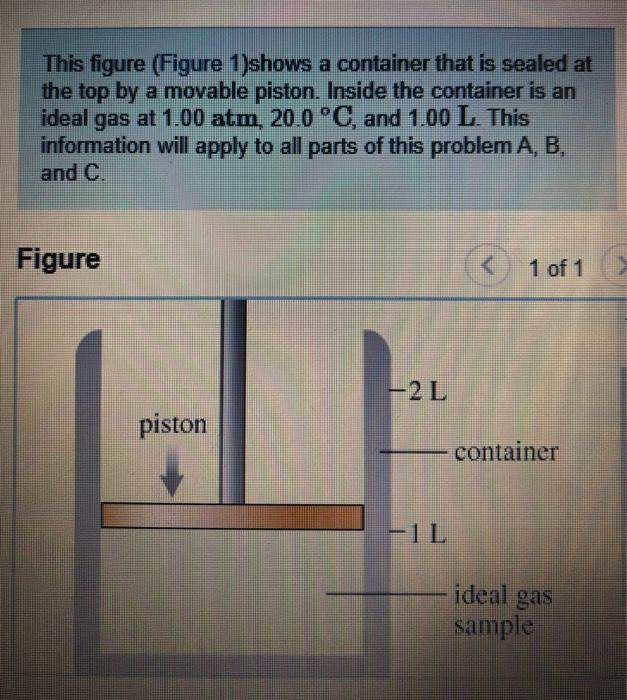
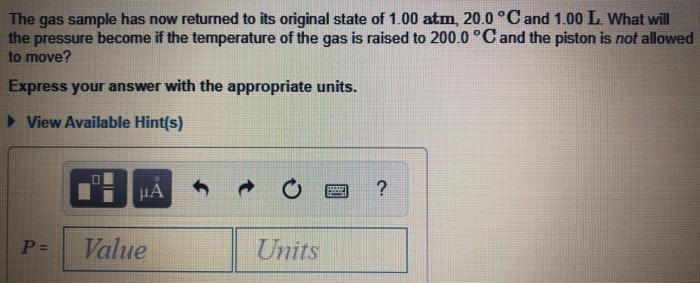
This figure (Figure 1)shows a container that is sealed at the top by a movable piston. Inside the container is an gas at 1.00 atm, 20.0 C, and 1.00 L. This information will apply to all parts of this problem A, B, and C. ideal Figure The gas sample has now returned to its original state of 1.00 atm, 20.0 C and 1.00 L What will the pressure become if the temperature of the gas is raised to 200.0 C and the piston is not allowed to move? Express your answer with the appropriate units. > View Available Hint(s) HA ? Value Units
Step by Step Solution
3.36 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


