Answered step by step
Verified Expert Solution
Question
1 Approved Answer
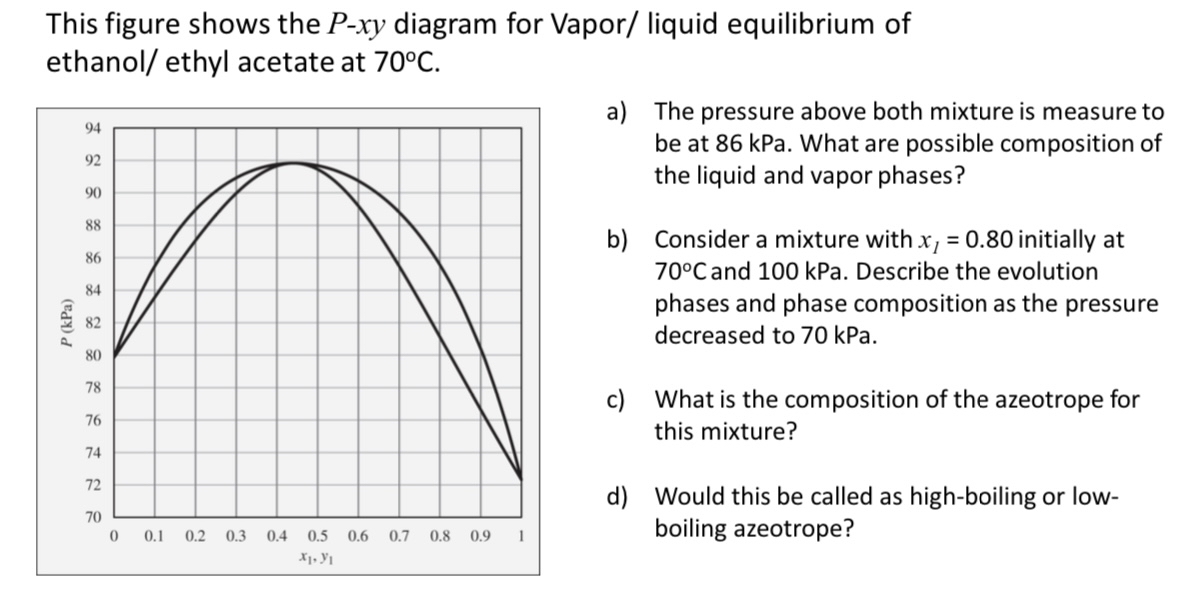
This figure shows the P - x y diagram for Vapor / liquid equilibrium of ethanol / ethyl acetate at 7 0 C . a
This figure shows the diagram for Vapor liquid equilibrium of ethanol ethyl acetate at
a The pressure above both mixture is measure to be at kPa. What are possible composition of the liquid and vapor phases?
b Consider a mixture with initially at and kPa. Describe the evolution phases and phase composition as the pressure decreased to kPa.
c What is the composition of the azeotrope for this mixture?
d Would this be called as highboiling or lowboiling azeotrope?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


