Answered step by step
Verified Expert Solution
Question
1 Approved Answer
This is a 1 question which is in the format of a flow chart with drop down boxes. Therefore I will take several photos to
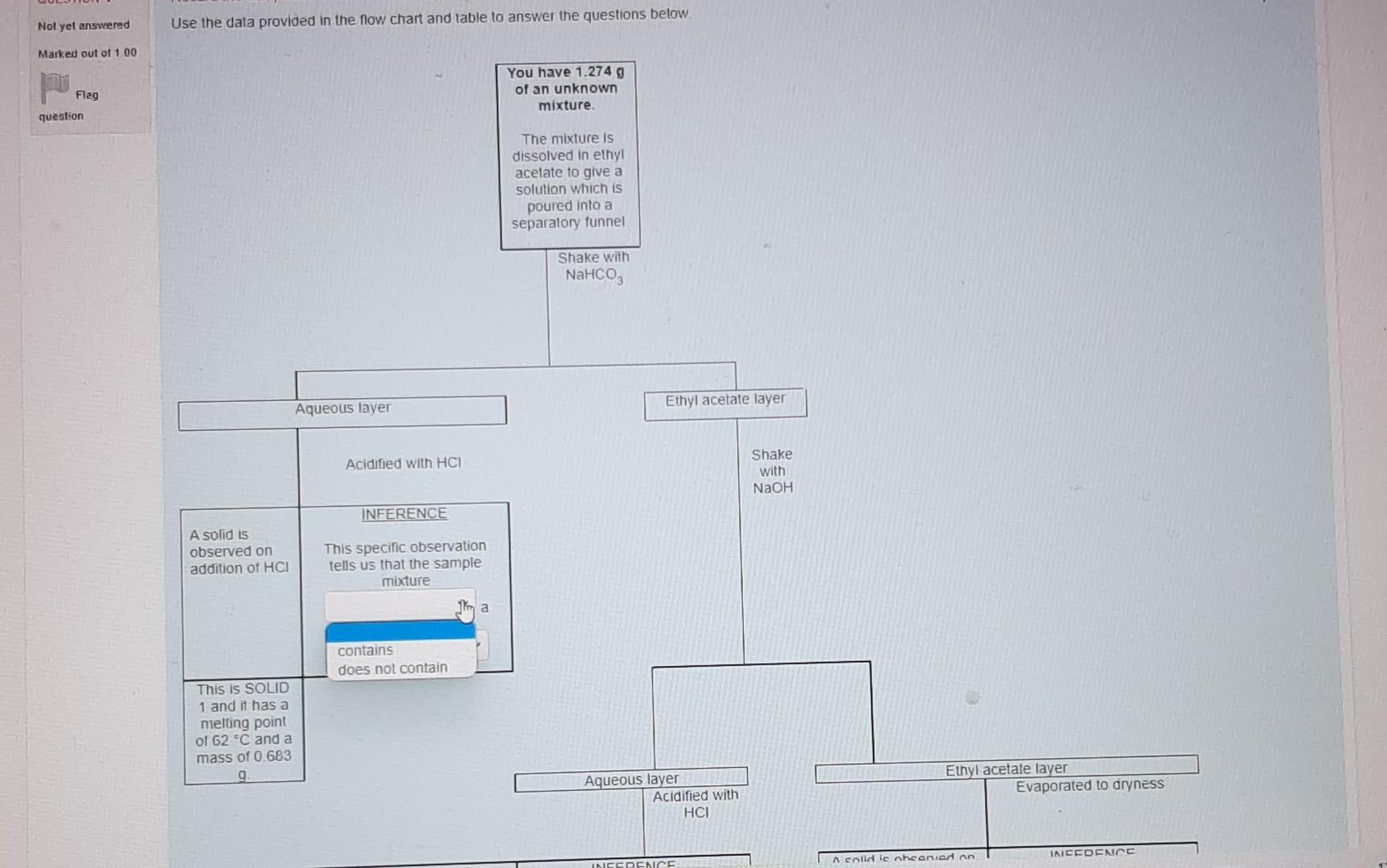
This is a 1 question which is in the format of a flow chart with drop down boxes. Therefore I will take several photos to show the options that are there to be selected as the answer. I appreciate any help offered. Thanks in advance
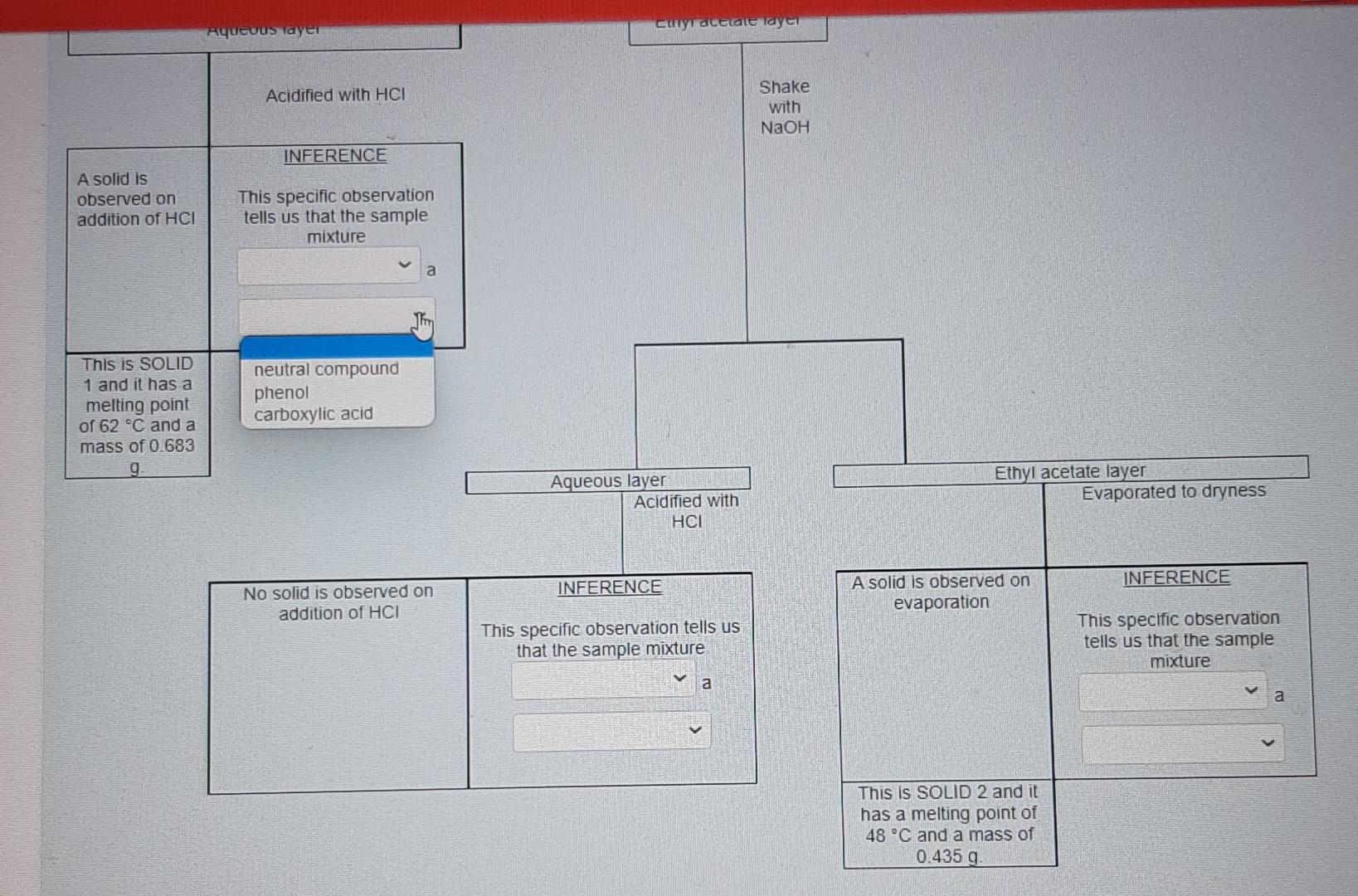
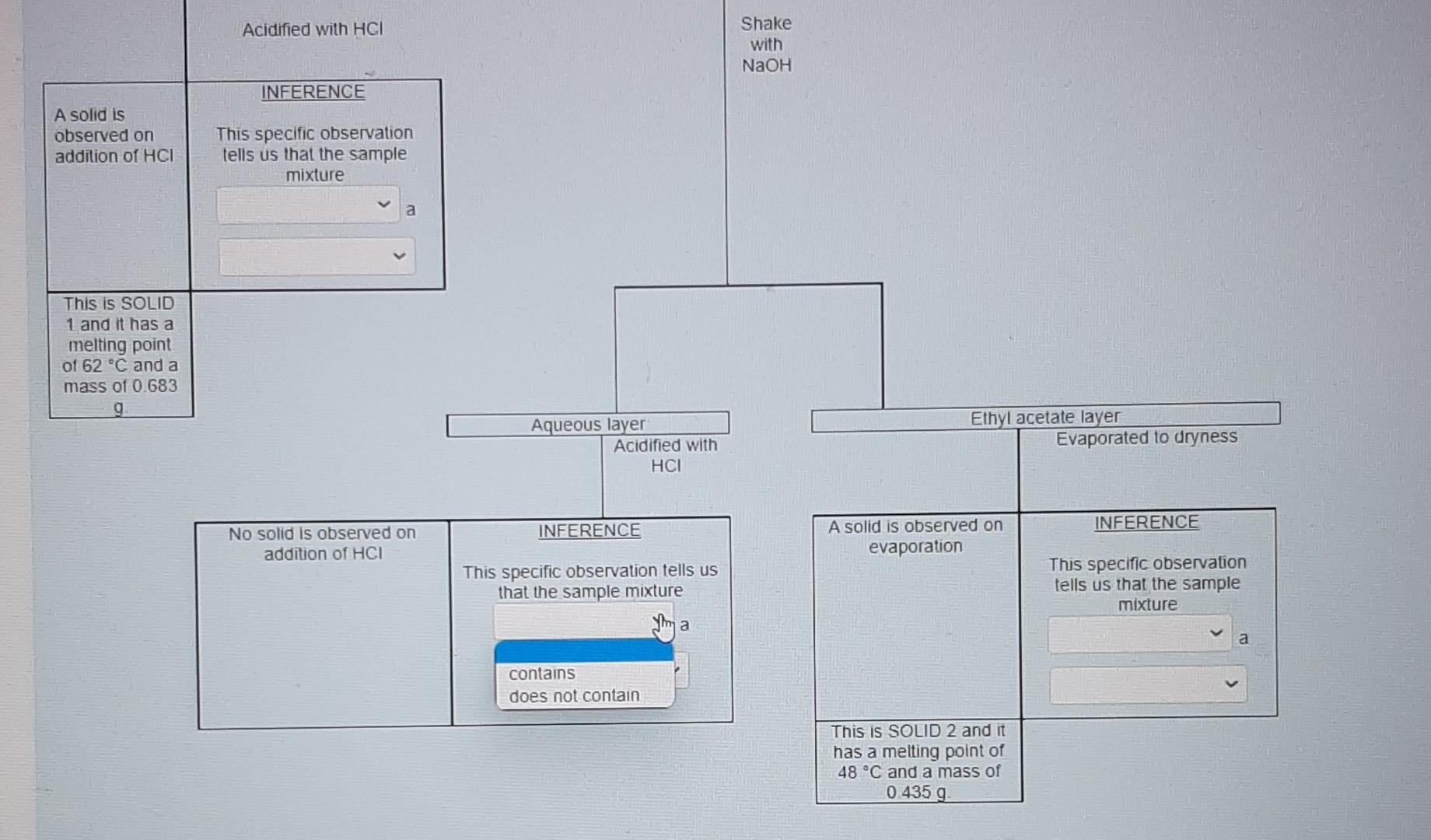
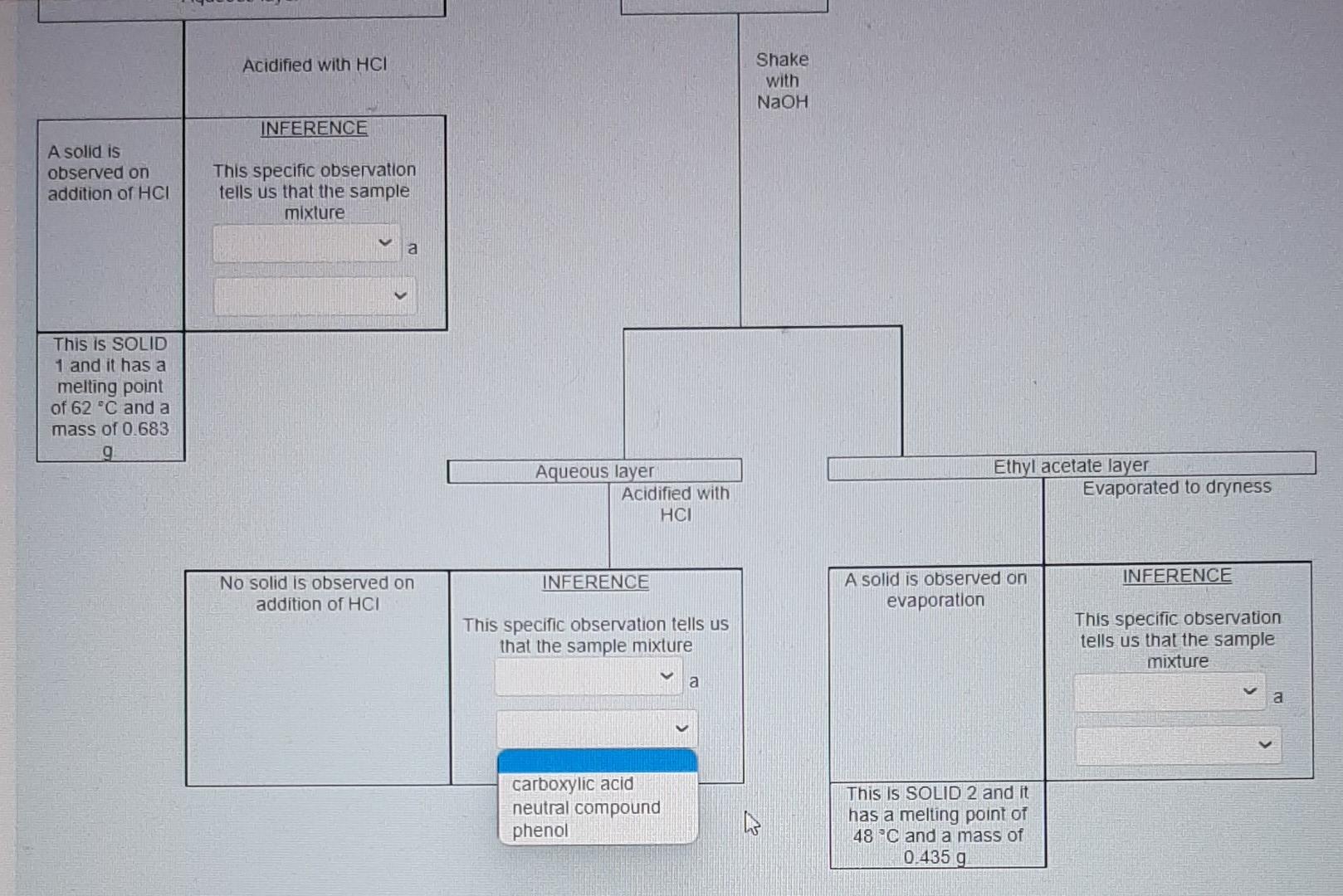
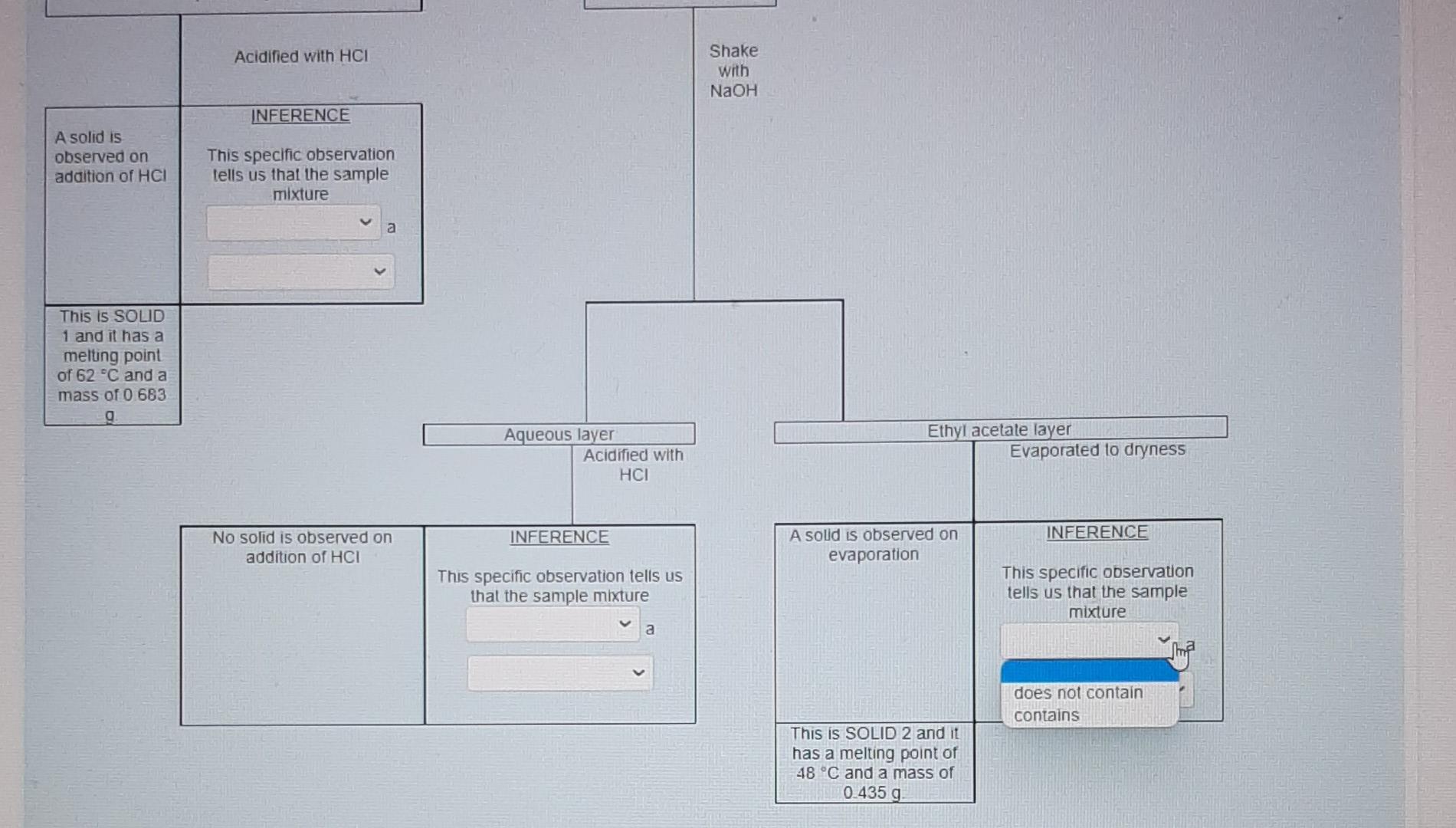
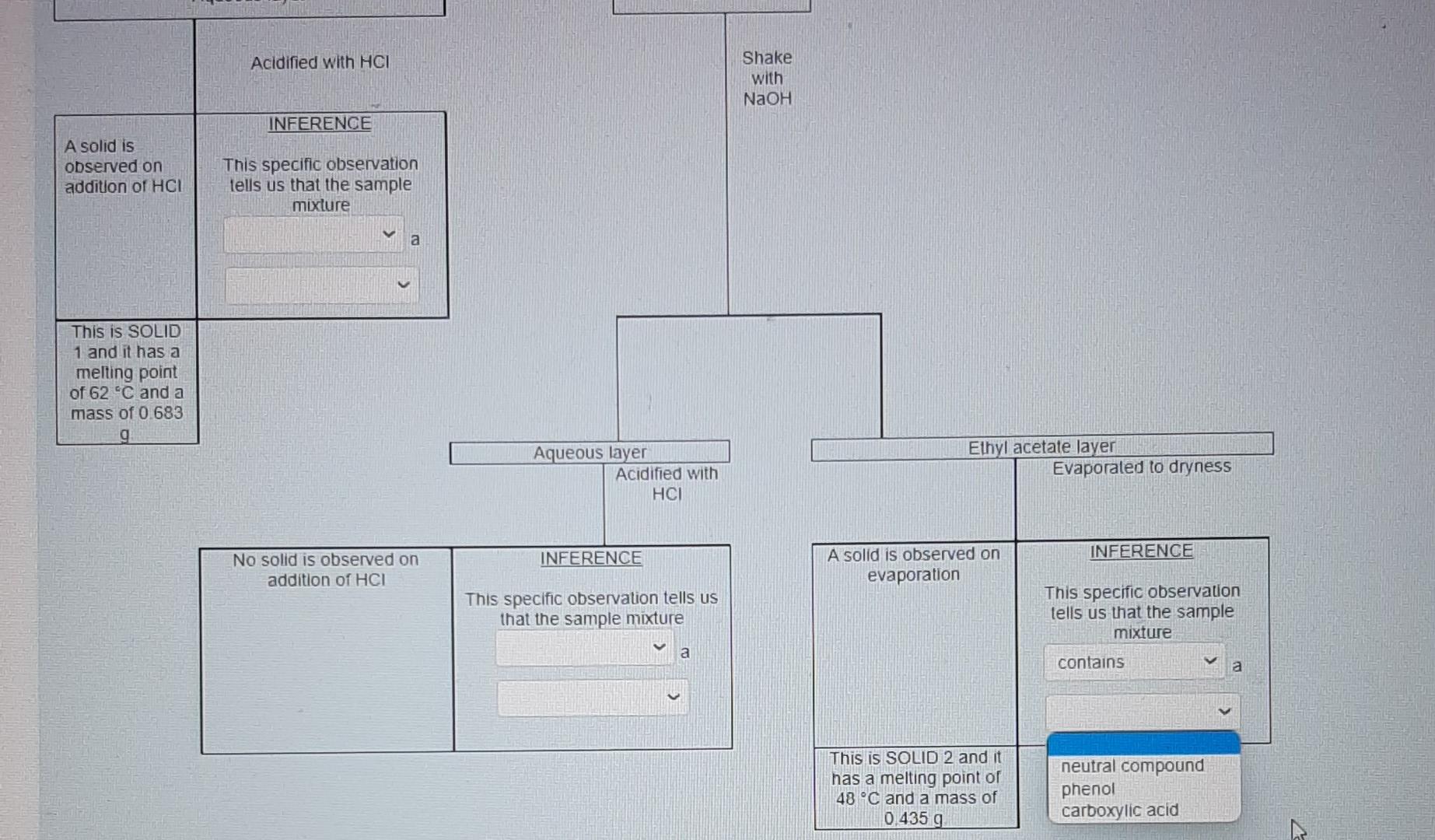
for this photo I accidentally selected "contain" for the one above therefore I am not sure if it is correct.
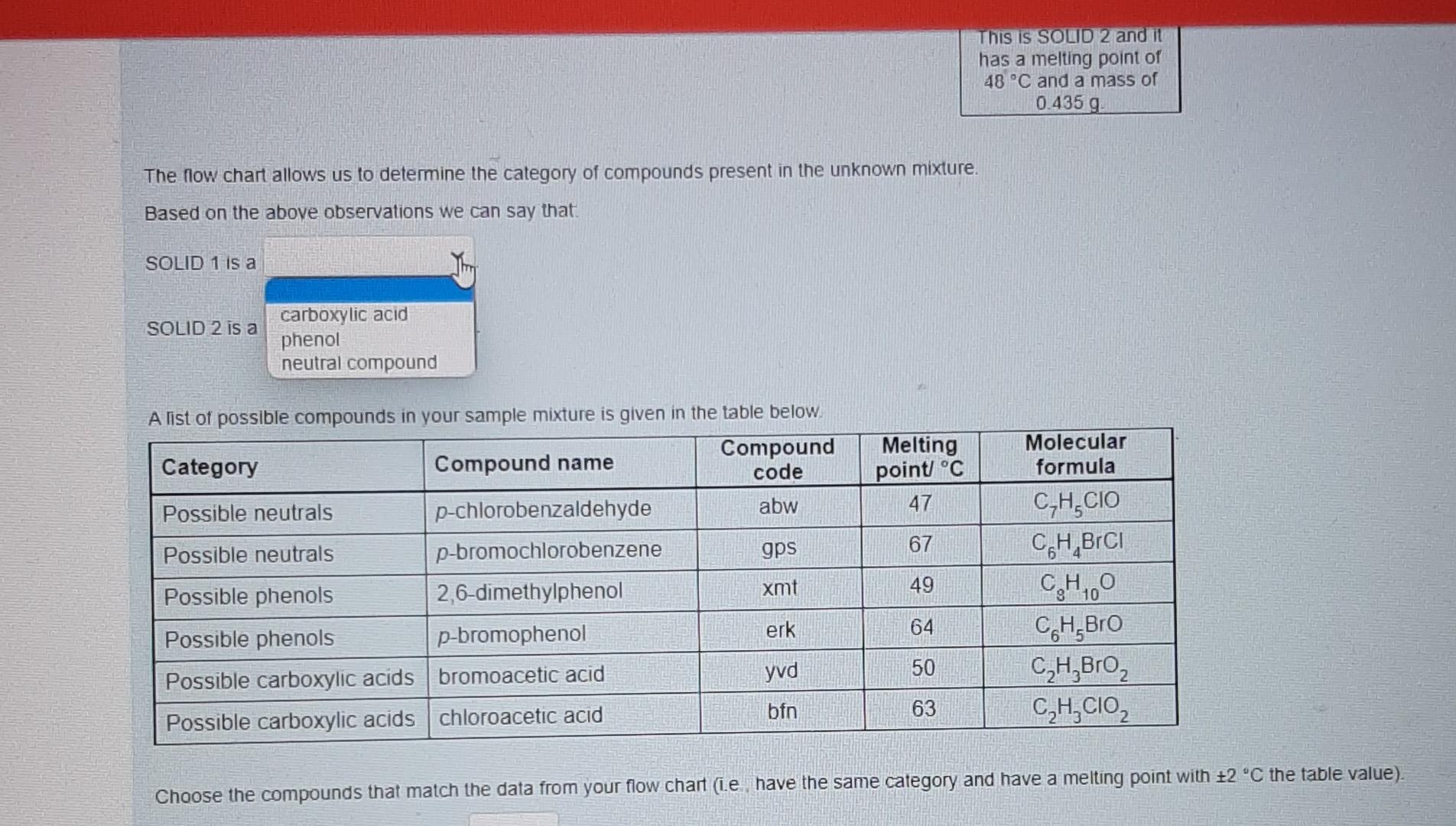
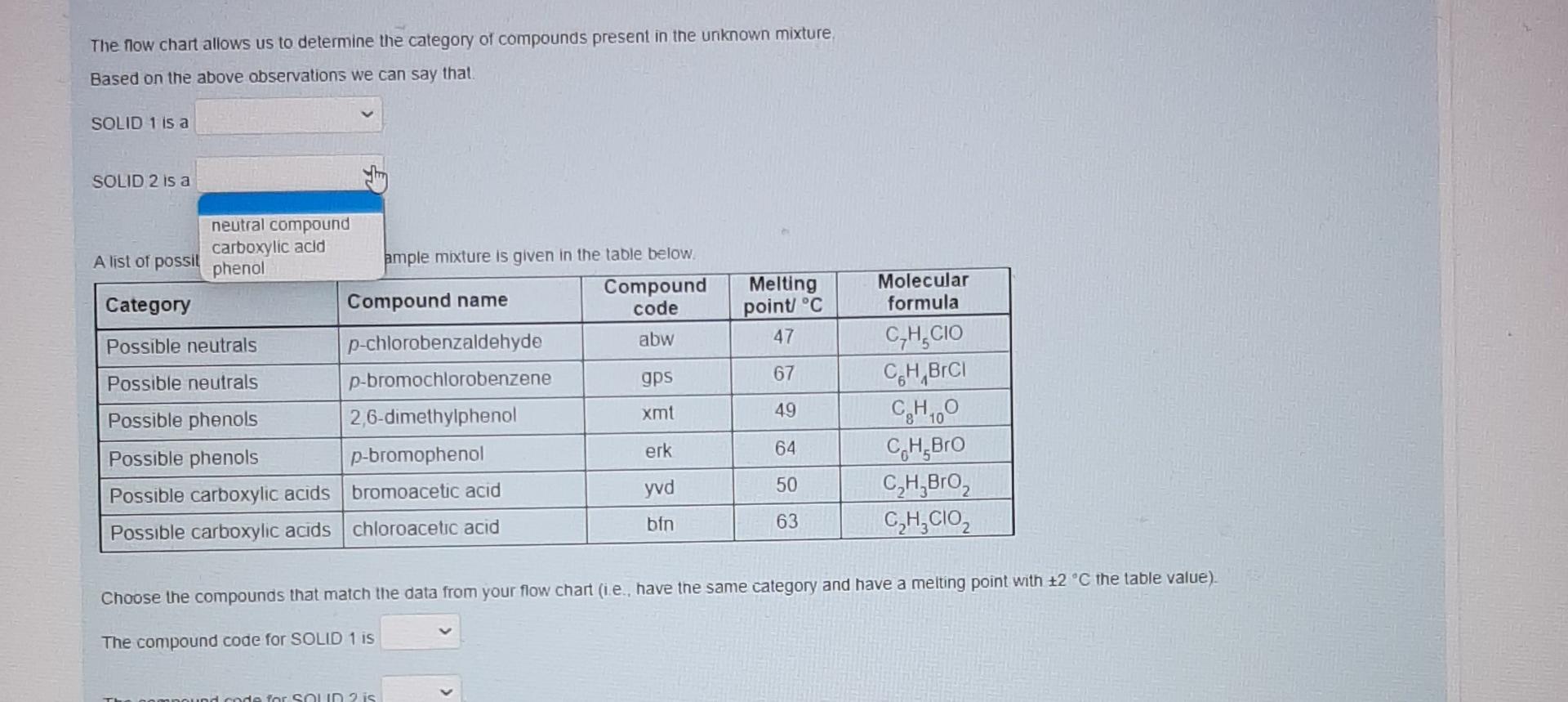
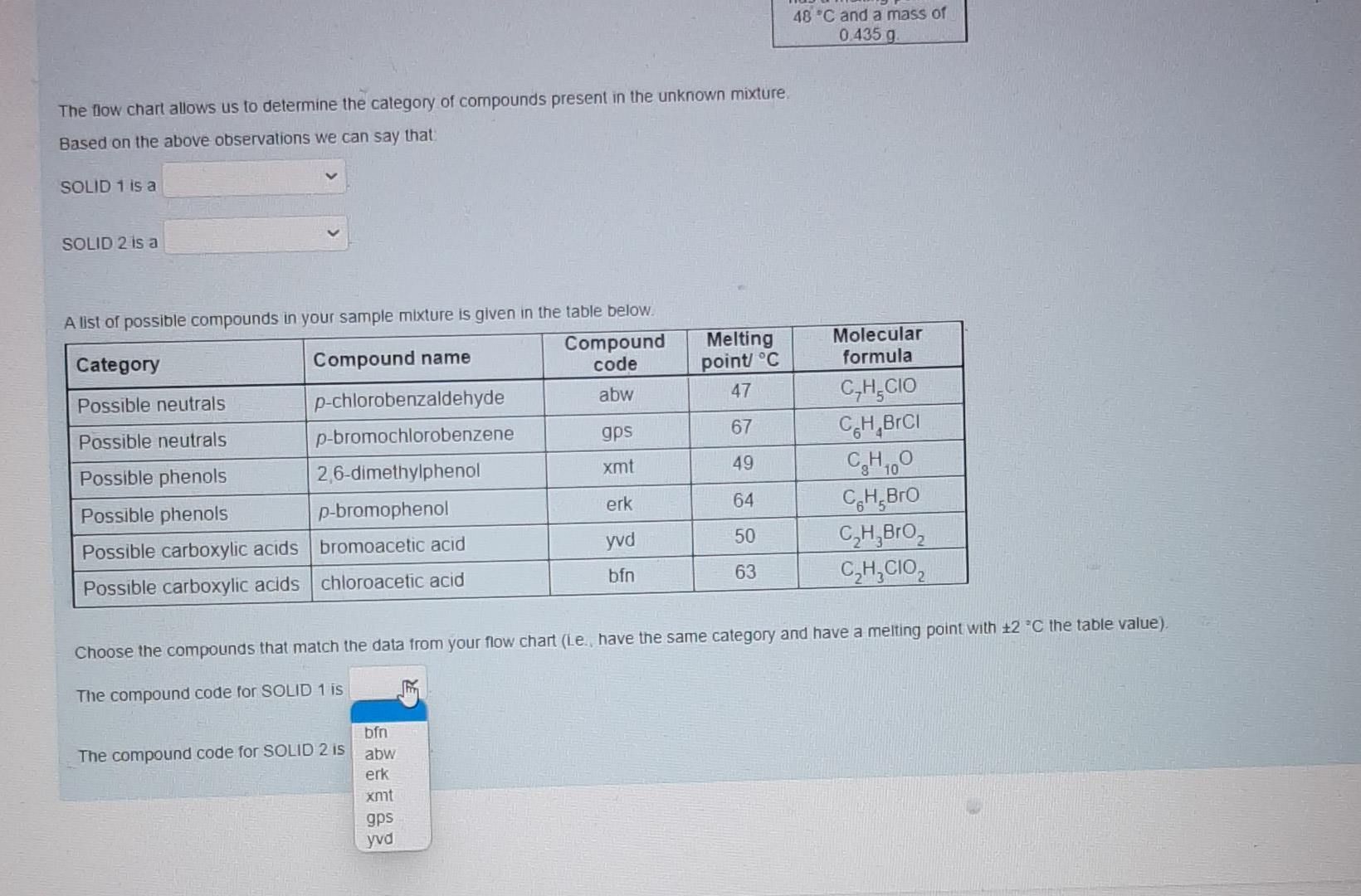
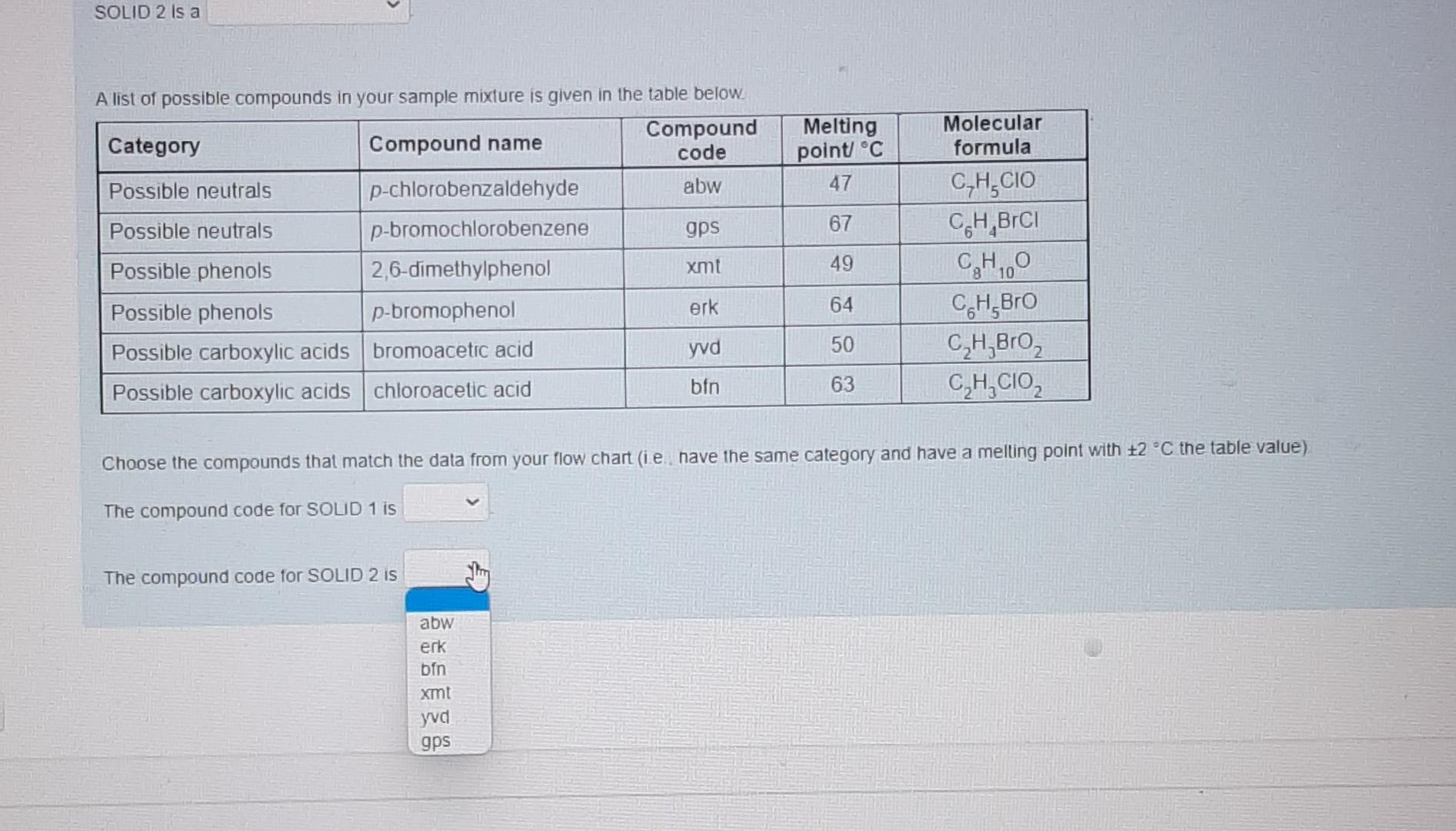
These are individual photos showing each drop down options.I hope it is not confusing. Thanks alot in advanced. Please respond as soon as possible.
Nol yel answered Use the data provided in the flow chart and table to answer the questions below Marked out of 1.00 Flag question You have 1.2749 of an unknown mixture. The mixture is dissolved in ethyl acetate to give a solution which is poured into a separatory funnel Shake with NaHCO, Ethyl acetate layer Aqueous layer Acidified with HCI Shake with NaOH INFERENCE A solid is observed on addition of HCI This specific observation tells us that the sample mixture Jim a contains does not contain This is SOLID 1 and it has a melting point of 62 C and a mass of 0.683 g Ethyl acetate layer Evaporated to dryness Aqueous layer Acidified with HCI INICCOENOC Acadienhsanier nn. INICERENCE Cu aceae cycl AQUEOUS layer Acidified with HCI Shake with NaOH INFERENCE A solid is observed on addition of HCI This specific observation tells us that the sample mixture a TI This is SOLID 1 and it has a melting point of 62 C and a mass of 0.683 g neutral compound phenol carboxylic acid Aqueous layer Acidified with HCI Ethyl acetate layer Evaporated to dryness INFERENCE INFERENCE No solid is observed on addition of HCI A solid is observed on evaporation This specific observation tells us that the sample mixture This specific observation tells us that the sample mixture a a This is SOLID 2 and it has a melting point of 48 C and a mass of 0.435 g Acidified with HCI Shake with NaOH INFERENCE A solid is observed on addition of HCI This specific observation tells us that the sample mixture a This is SOLID 1 and it has a melting point of 62 C and a mass of 0.683 g Aqueous layer Acidified with HCI Ethyl acetate layer Evaporated to dryness INFERENCE INFERENCE No solid is observed on addition of HCI A solid is observed on evaporation This specific observation tells us that the sample mixture This specific observation tells us that the sample mixture a a contains does not contain This is SOLID 2 and it has a melting point of 48 C and a mass of 0.435 g Acidified with HCI Shake with NaOH INFERENCE A solid is observed on addition of HCI This specific observation tells us that the sample mixture a This is SOLID 1 and it has a melting point of 62 C and a mass of 0.683 9 Aqueous layer Acidified with HCI Ethyl acetate layer Evaporated to dryness INFERENCE INFERENCE No solid is observed on addition of HCI A solid is observed on evaporation This specific observation tells us that the sample mixture This specific observation tells us that the sample mixture a a carboxylic acid neutral compound phenol This is SOLID 2 and it has a melting point of 48 C and a mass of 0.435 g & Acidified with HCI Shake with NaOH INFERENCE A solid is observed on addition of HCI This specific observation tells us that the sample mixture a This is SOLID 1 and it has a melting point of 62 C and a mass of 0 683 9 Aqueous layer Acidified with HCI Ethyl acetale layer Evaporated to dryness INFERENCE INFERENCE No solid is observed on addition of HCI A solid is observed on evaporation This specific observation tells us that the sample mixture This specific observation tells us that the sample mixture a Tho does not contain contains This is SOLID 2 and it has a melting point of 48 C and a mass of 0_435 g Acidified with HCI Shake with NaOH INFERENCE A solid is observed on addition of HCI This specific observation tells us that the sample mixture a This is SOLID 1 and it has a melting point of 62 C and a mass of 0.683 9 Aqueous layer Acidified with HCI Ethyl acetate layer Evaporaled to dryness INFERENCE INFERENCE No solid is observed on addition of HCI A solid is observed on evaporation This specific observation tells us that the sample mixture This specific observation tells us that the sample mixture contains a a This is SOLID 2 and it has a melting point of 48 C and a mass of 0.435 g neutral compound phenol carboxylic acid This is SOLID 2 and it has a melting point of 48 C and a mass of 0.435 g The now chart allows us to determine the category of compounds present in the unknown mixture. Based on the above observations we can say that SOLID 1 is a SOLID 2 is a carboxylic acid phenol neutral compound Melting point C 47 67 A list of possible compounds in your sample mixture is given in the table below. Category Compound name Compound code Possible neutrals p-chlorobenzaldehyde abw Possible neutrals p-bromochlorobenzene gps Possible phenols 2,6-dimethylphenol xmt Possible phenols p-bromophenol erk Possible carboxylic acids bromoacetic acid yud Possible carboxylic acids chloroacetic acid bfn 49 Molecular formula C,H.CIO C.H.BICI CH, CH, Bro CH,Broz C,H, CO2 64 50 63 Choose the compounds that match the data from your flow chant (le have the same category and have a melting point with +2 C the table value) The low chart allows us to determine the category of compounds present in the unknown mixture Based on the above observations we can say that SOLID 1 is a SOLID 2 is a Melting point C 47 67 neutral compound A list of possil carboxylic acid phenol ample mixture is given in the table below. Compound Category Compound name code Possible neutrals p-chlorobenzaldehyde abw Possible neutrals p-bromochlorobenzene gps Possible phenols 2,6-dimethylphenol xmt Possible phenols p-bromophenol erk Possible carboxylic acids bromoacetic acid yvd Possible carboxylic acids chloroacetic acid bin 49 Molecular formula C,H, CIO CH,BICI CHO CH.Bro C,HBroz C,H, CIOZ 64 50 63 Choose the compounds that match the data from your flow chart (ie., have the same category and have a melting point with +2 C the table value) The compound code for SOLID 1 is code for SOLIDS is 48 C and a mass of 0.435 9 The low chart allows us to determine the category of compounds present in the unknown mixture Based on the above observations we can say that SOLID 1 is a SOLID 2 is a Melting point C 47 67 A list of possible compounds in your sample mixture is given in the table below. Category Compound name Compound code Possible neutrals p-chlorobenzaldehyde abw Possible neutrals p-bromochlorobenzene gps Possible phenols 26-dimethylphenol xmt Possible phenols p-bromophenol erk Possible carboxylic acids bromoacetic acid yud Possible carboxylic acids chloroacetic acid bfn 49 Molecular formula CHECIO C.H.BrCI CHO CH, Bro CH, Broz CH,CIOZ 64 50 63 Choose the compounds that match the data from your flow chart (Le have the same category and have a melting point with +2 C the table value) The compound code for SOLID 1 is The compound code for SOLID 2 is bin abw erk xmt gps yud SOLID 2 Is a Melting point/ C 47 67 A list of possible compounds in your sample mixture is given in the table below. Category Compound Compound name code Possible neutrals p-chlorobenzaldehyde abw Possible neutrals p-bromochlorobenzene gps Possible phenols 2,6-dimethylphenol xmt Possible phenols p-bromophenol erk Possible carboxylic acids bromoacetic acid yud Possible carboxylic acids chloroacetic acid bfn 49 Molecular formula C,H,CIO CH,BICI CH, CH, Bro C,H, Broz C,H,CIOZ 10 64 50 63 Choose the compounds that match the data from your flow chart (i e have the same category and have a melting point with +2 C the table value) The compound code for SOLID 1 is The compound code for SOLID 2 is abw erk bin xmt yud gpsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


