Question
This is an exercise taken from Welty's book, but I didn't understand the mass balance used in the solution. Please explain to me step by
This is an exercise taken from Welty's book, but I didn't understand the mass balance used in the solution.
Please explain to me step by step.
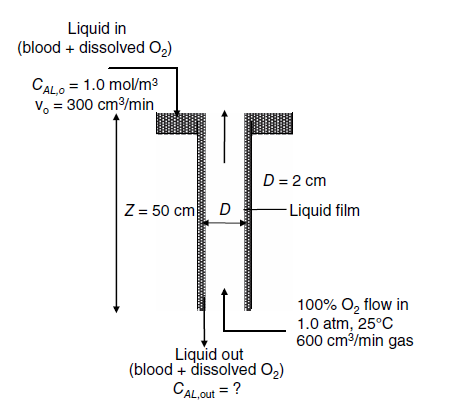
A wetted-wall column of 2.0-cm inner diameter and 50-cm wetted length is used to oxygenate blood as a continuous, steady-state process, as shown in the figure below. Blood containing 1.0 gmole/m3 of dissolved oxygen enters the top of the wetted-wall column at a volumetric flow rate of 300 cm3/min. Pure, 100% O2 gas at 1.0 atm and 25C enters the bottom of the column at a volumetric gas flow rate of 600 cm3/min. A very simplified description for estimating the equilibrium solubility of O2 dissolved in blood is
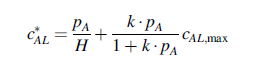
where pA is the partial pressure of O2 in the gas phase, H =0.8 atm m3/gmole for O2 in the blood plasma, k= 28 atm-1 for the blood hemoglobin, and cAL,max =9.3 gmole O2/m3 for the hemoglobin.
At 25C, the kinematic viscosity of blood is 0.040 cm2/s and the density of blood is 1.025 g/cm3. You may assume that the diffusion coefficient of O2 in blood approximates the diffusion coefficient of O2 in liquid water, which is 2.0 x10-5 cm2/s at 25C.
 a. What is the mass-transfer coefficient for O2 into the flowing liquid film?
a. What is the mass-transfer coefficient for O2 into the flowing liquid film?
b. What is the concentration of dissolved oxygen in the liquid exiting the bottom of the column, cAL,out?
cAL=HpA+1+kpAkpAcAL,max Liquid in (blood + dissolved O2 )
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


