Answered step by step
Verified Expert Solution
Question
1 Approved Answer
This is Crystal Violet Lab of Ap Chemistry Please help me on 1,2,3,4,5 Here is the data table 1. Was the reaction zero, first, or
This is Crystal Violet Lab of Ap Chemistry 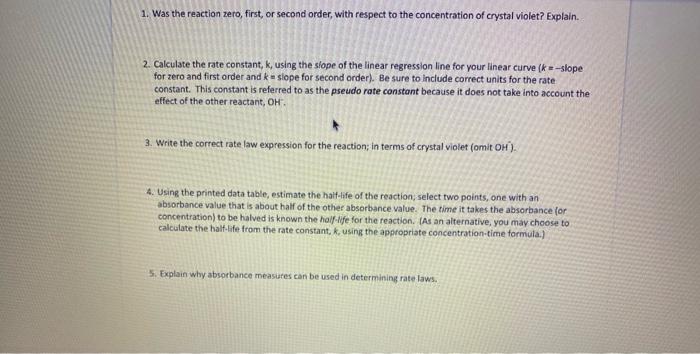
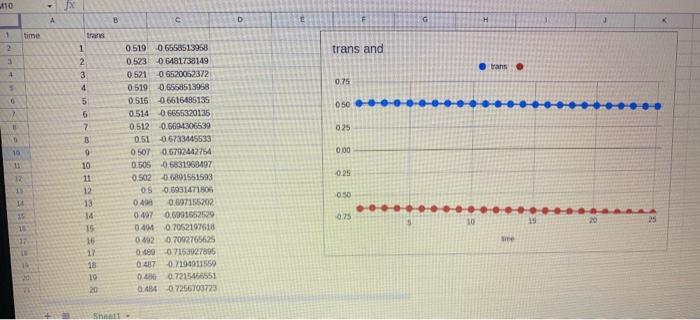
1. Was the reaction zero, first, or second order, with respect to the concentration of crystal violet? Explain. 2. Calculate the rate constant, k, using the siope of the linear regression line for your linear curve (k=slope for zero and first order and k= slope for second order). Be sure to include correct units for the rate constant. This constant is referred to as the pseudo rate constant because it does not take into account the effect of the other reactant, OH. 3. Write the correct rate law expression for the reaction; in terms of crystal violet (omit OH ). 4. Using the printed data table, estimate the half-ife of the reaction; select two points, one with an absorbance value that is about half of the other absorbance value. The time it takes the absorbance for concentration) to be halved is known the haif-ilfe for the reaction. (As an alternative, you may choose to calculate the half-ife from the rate constant, k, using the appropriate concentration-time formula.) 5. Explain why absorbance measures can be used in determining rate laws. 1. Was the reaction zero, first, or second order, with respect to the concentration of crystal violet? Explain. 2. Calculate the rate constant, k, using the siope of the linear regression line for your linear curve (k=slope for zero and first order and k= slope for second order). Be sure to include correct units for the rate constant. This constant is referred to as the pseudo rate constant because it does not take into account the effect of the other reactant, OH. 3. Write the correct rate law expression for the reaction; in terms of crystal violet (omit OH ). 4. Using the printed data table, estimate the half-ife of the reaction; select two points, one with an absorbance value that is about half of the other absorbance value. The time it takes the absorbance for concentration) to be halved is known the haif-ilfe for the reaction. (As an alternative, you may choose to calculate the half-ife from the rate constant, k, using the appropriate concentration-time formula.) 5. Explain why absorbance measures can be used in determining rate laws Please help me on 1,2,3,4,5

Here is the data table

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


