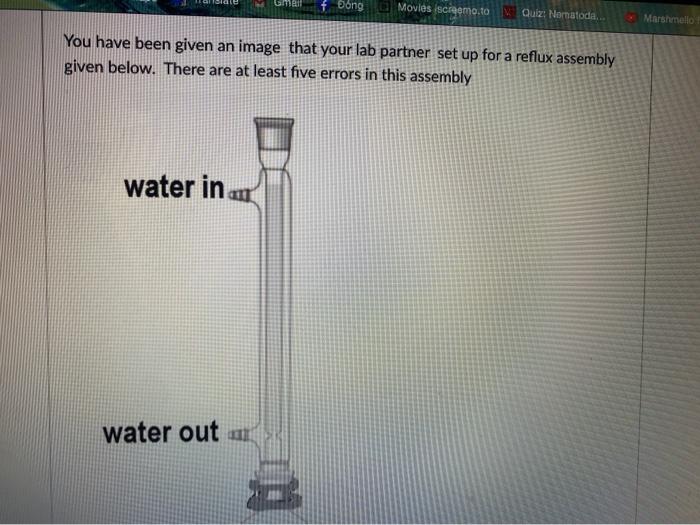
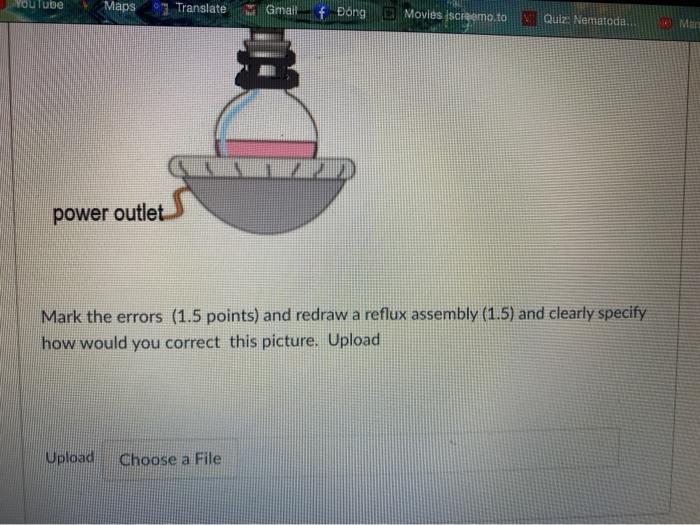
This is organic chemistry 2 , I need the best answer for this question to prepare my first lab exam. Thank you so much for helping me.
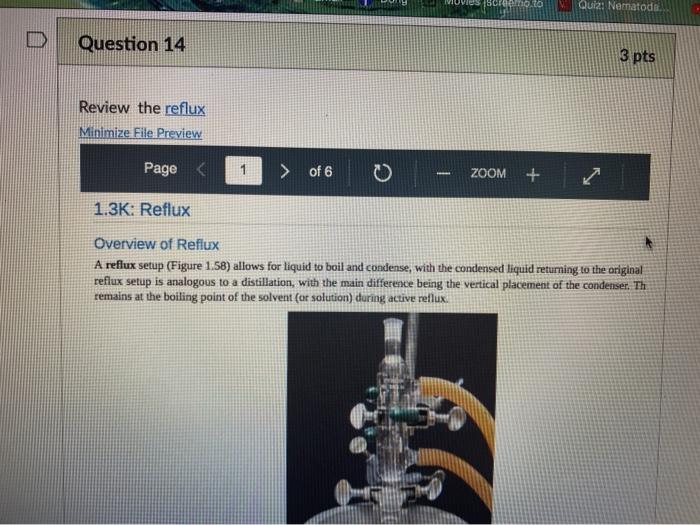
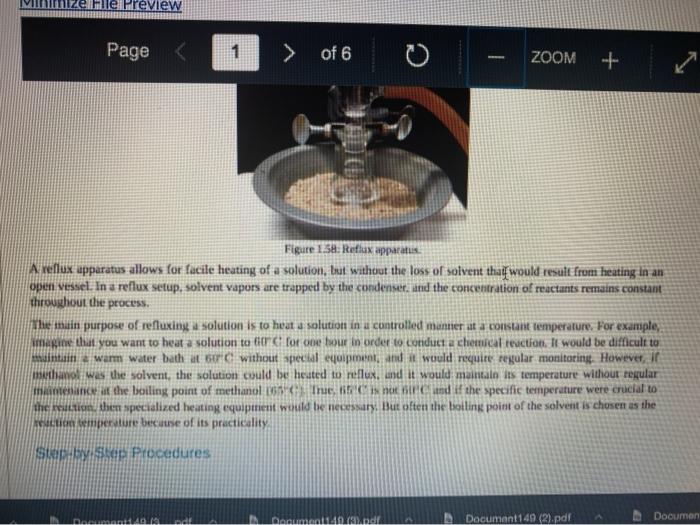
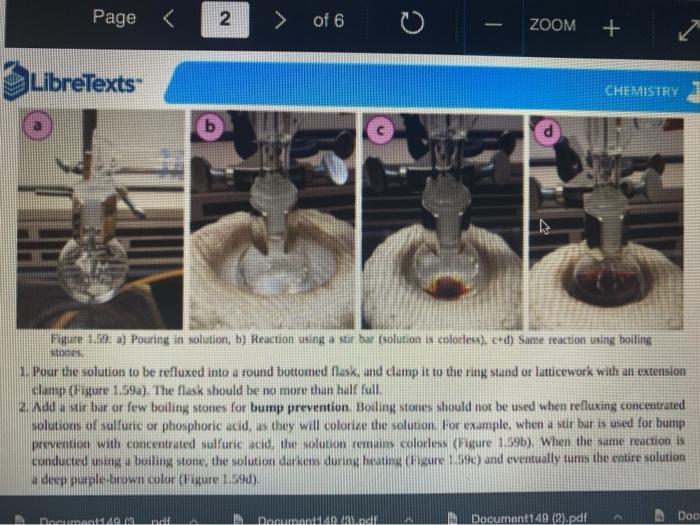
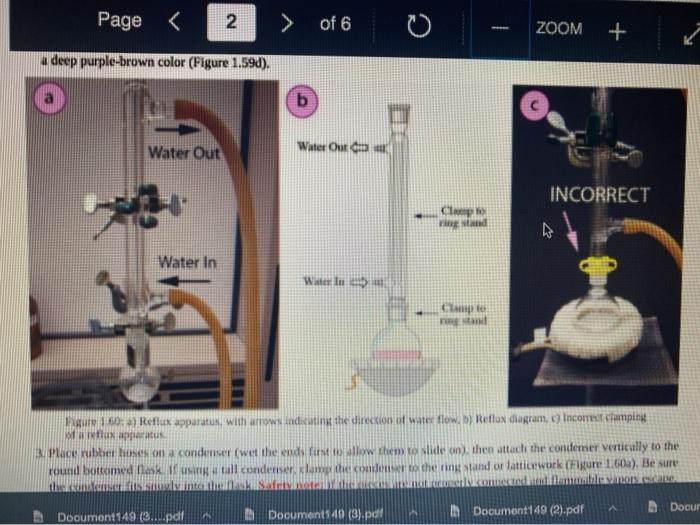
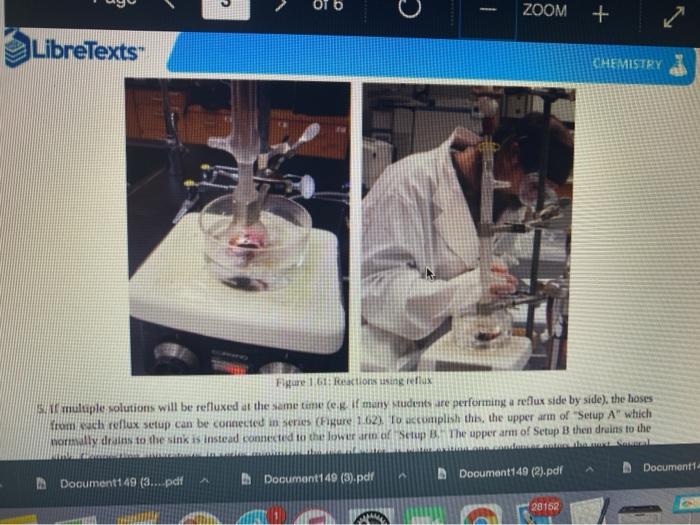
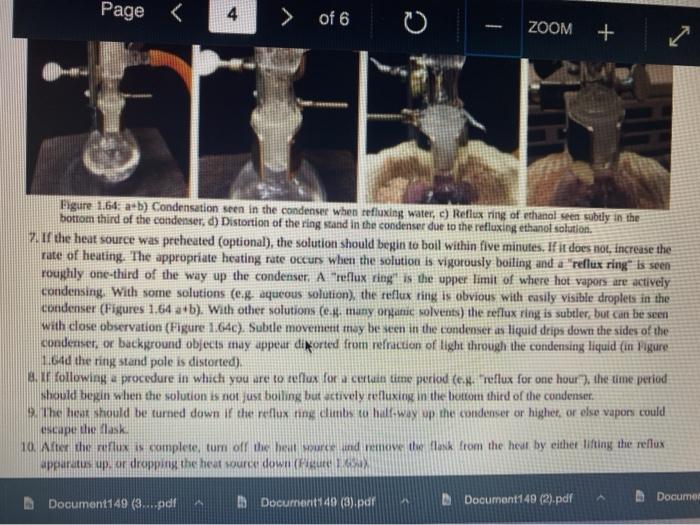
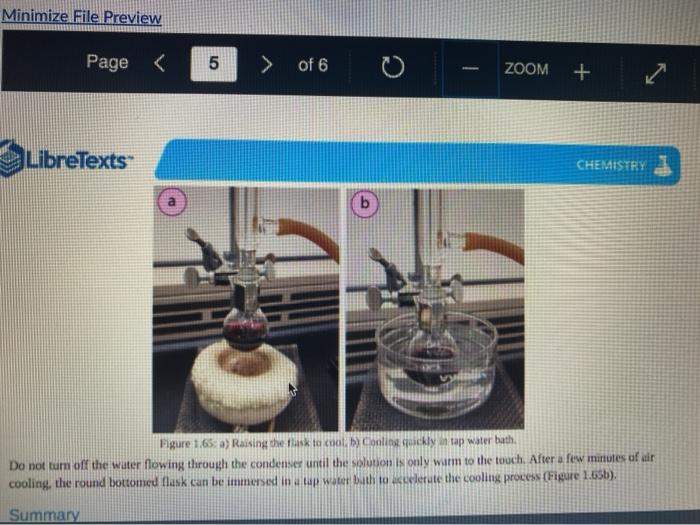
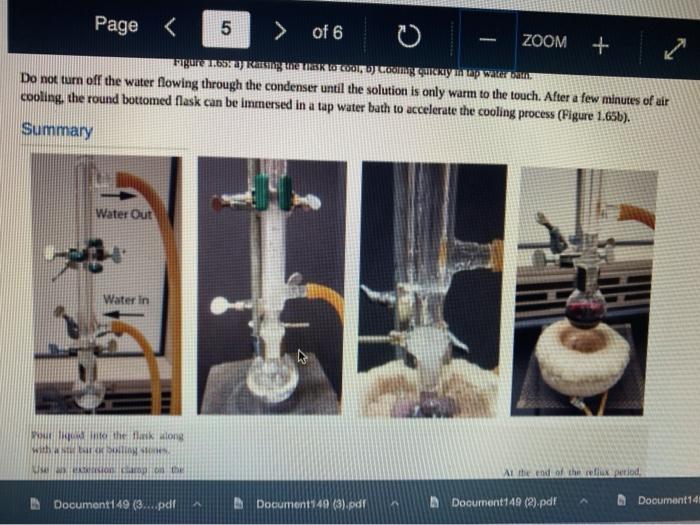
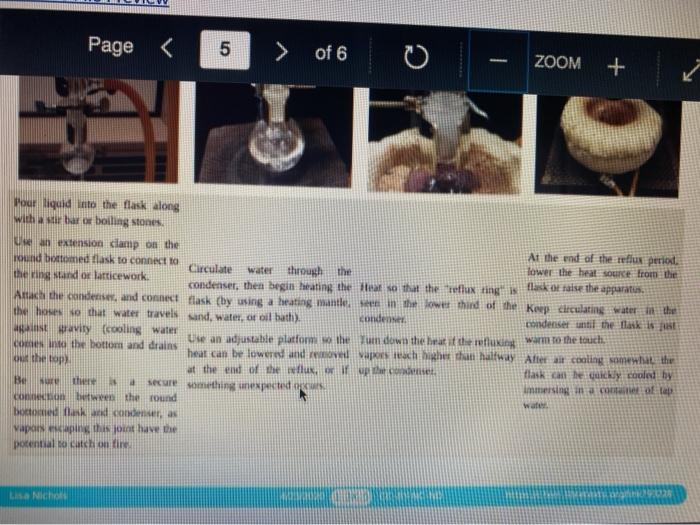
Quiz! Nematoda Question 14 3 pts Review the reflux Minimize File Preview Pages > of 6 ZOOM + 1.3K: Reflux Overview of Reflux A reflux setup (Figure 1.58) allows for liquid to boil and condense, with the condensed liquid returning to the original reflux setup is analogous to a distillation, with the main difference being the vertical placement of the condenser. Th remains at the boiling point of the solvent (or solution) during active reflux. Preview Page of 6 ZOOM ---- Figure 1.5: Reflux apparatus Ad reflux apparatus allows for facile heating of a solution, but without the loss of solvent that would result from heating in an open vessel. In a reflux setup, solvent vapors are trapped by the condenser and the concentration of reactants remains constant throughout the process The main purpose of refluxing a solution is to hwat a solutions in a controlled munner at a constant temperature. For example, magine that you want to hear a solution to or for one tour in order to conduct a chemical reaction. It would be difficult to maintain = warm water bath at without special equipment, and it would require regular monitoring. However, if thanol was the solvent, the solution could be heuted to reflux. and it would maintain its temperature without regular intenance at the boiling point of methanol 1) True, I HE BEC ind of the specific tenperature were crucial to the multion, then specialized heating equipment would be necessary. But often the botling point of the solvent is chosen as the Realition semperature because of its practicality Step by Step Procedures No od Document140 Documen Document149 (2).pdf Page N > of 6 ZOOM + LibreTexts CHEMISTRY Figure 1.59. a) Pouring in solution, b) Reaction using a star bar isolution in colorless), cc) Same reaction using boiling Stones 1. Pour the solution to be refluxed into a round bottomed flask, and clamp it to the ring stand or latticework with an extension clamp (Figure 1.59a). The flask should be no more than half full, 2. Add a stir bar or few boiling stones for bump prevention Boiling stones should not be used when refluxing concentrated solutions of sulfuric or phosphoric acid, as they will colorize the solution. For example, when a stir bar is used for bump prevention with concentrated sulfuric acid the solution remains colorless (Figure 1.59b) When the same reaction is conducted using a boiling stone, the solution durken during heating gure 1.59c) and eventually tums the entire solution a deep purple brown color (figure 59). NGGA or Deum ad Document149 (2).pdf Dod Page of 6 200M + a deep purple-brown color (Figure 1.59d). b Water Out Water Onam INCORRECT Campo ning sind Water In Waterlu Canto sur 60: a) Reflux apparatus, with arrow indicating the direction of water flow.) Reflux diagram,Incorrect clamping un apparatus 3. Place rubber hoses on a condenser (wet the ends into allow them to slide on), then attach the condemer vertically to the round bottomed ask using a tall condenser Kamp dhe kondenser to the ring stand or lattice work (Figure 1.60a). Be sure nunta emblea D Docur Documont 140 (3....pdf Document140 (3).pdf Document149 (2).pdf Minimize File Preview Page of 6 ZOOM Figure 1.60: a) Reflux apparatus, with arrows indicating the direction of water flow, b) Reflux diagram, c) Incorrect clamping of a reflux apparatus 8. Place rubber hoses on a condenser (wet the ends first to allow them to slide on), then attach the condenser vertically to the round bottomed flask. If using a tall condenser, clamp the condenser to the ring stand or latticework (Figure 1.60). Be sure the condenser fits snugly into the flask. Safety note: if the pieces are not properly connected and flammable vapors escape, they may be ignited by the heat source. Do not connect the round bottomed Nask and condenser with a plastic dip, as shown in Figure 1.60e. Plastic clips can sometimes fail (especially when they are heated), and this setup does not allow for the flask to be reliably removed from the heat source at the end of the reflux. A Connect the hose on the lower arm of the condenser to the water faucet and allow the base on the upper arm to drain to the sink (Figure 1.60b). It is important that water goes in the bottom of the condenser and out the top (se water flows against kruvity) or else the condenser will be ineffective as it will not fill completely. . liteit 3 C ---- ZOOM + LibreTexts CHEMISTRY Flane 1.61: Reactions using reflex multiple solutions will be refluxed at the same time if many students are performing a reflux side by side), the hoses frontuch reflux setup can be connected in series (Figure 1.62) To accomplish this, the upper arm of "Setup A" which normally drains to the sink is instead connected to the lower arm of Setup B. The upper arm of Setup B then drains to the ES WAN Document1 Document1 49 (3....pdf Document149 (3).pdf Dooument149 (2).pdf 28162 Page of 6 O ZOOM + TRONICS 5. If multiple solutions will be refluxed at the same time (e.g. if many students are performing a reflux side by side), the hoses from each reflux setup can be connected in series (Figure 1.62). To accomplish this, the upper arm of "Setup A" which normally drains to the sink is instead connected to the lower arm of "Setup B.' The upper arm of Setup B then drains to the sink. Connecting apparatuses in series minimizes the use of water, as water exiting one condenser enters the next. Several reflux setups can be connected in series, and the water flow should be monitored to ensure that all setups are adequately cooled. Setup B Setup A to drain to B frorilaucet Figure 1.62: Kinecting flux condensers series 6 Bayan circumstances in that the disclops around from the high water Docum Daman Document149/2).pdf Docum Page of 6 ZOOM + Figure 1.66: a-b) Condensation seen in the condensee when refluxing water, e) Reflux ring of ethanol seen subtly in the bottom third of the condenser, d) Distortion of the ring stand in the condenser due to the refluxing ethanol solution. 2. If the heat source was preheated (optional), the solution should begin to boil within five minutes. If it does not, increase the rate of heuting. The appropriate heating rate occurs when the solution is vigorously boiling and a "reflux ring" is seen roughly one-third of the way up the condenser: A reflux ring" is the upper limit of where hot vapors are actively condensing. With some solutions (eg. aqueous solution) the reflux ring is obvious with easily visible droplets in the condenser (Figures 1.64 a+b). With other solutions (e.ff. many organic solvents) the reflux ring is subtler, but can be seen with close observation (Figure 1.64c). Subtle movement may be seen in the condenser as liquid deips down the sides of the condenser, or background objects may appear diported from refraction of light through the condensing liquid (in Figure 164d the ring stand pole is distorted). 8. f following a procedure in which you are to reflux fora certains Litme period (e.4. reflux for one hour"), the time period should begin when the solution is not just boiling but actively refluxing in the bottom third of the condenser. 9. The best should be turned down if the reflux ting dits to half way up the condenser or highet, or else vapors could escape the task 10. After the reflux is complete turn off the best source and the task from the heat by either lifting the reflux apparatus up. or dropping the heat source down (Part Document149 (3....pdf Document140 (3).pdf Document149 (2).pdf Documen Minimize File Preview Page 5 of 6 ZOOM + LibreTexts CHEMISTRY b Figure 1.65: a) Raising the task to coot by Cooling quickly an tap water bath Do not turn off the water flowing through the condenser until the solution is only warm to the touch. After a few minutes of air cooling, the round bottomed flask can be immersed in a tap water bath tolerute the cooling process (Figure 1.55b). Summary Page