Answered step by step
Verified Expert Solution
Question
1 Approved Answer
This is th question.Read it carefully see the figure attached for full data solve it correctly all answers related to it are incorrect .Show your

 This is th question.Read it carefully see the figure attached for full data solve it correctly all answers related to it are incorrect .Show your skills and i will rate u with best feedback.
This is th question.Read it carefully see the figure attached for full data solve it correctly all answers related to it are incorrect .Show your skills and i will rate u with best feedback.
I have attached answers at last so u may match yur answer before solution
thanks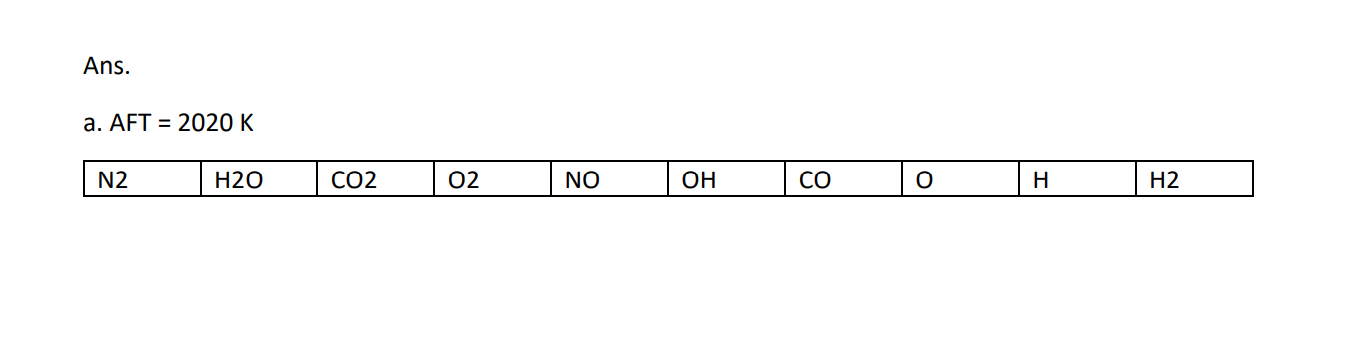
 I repeat again do it carefully and correctly
I repeat again do it carefully and correctly
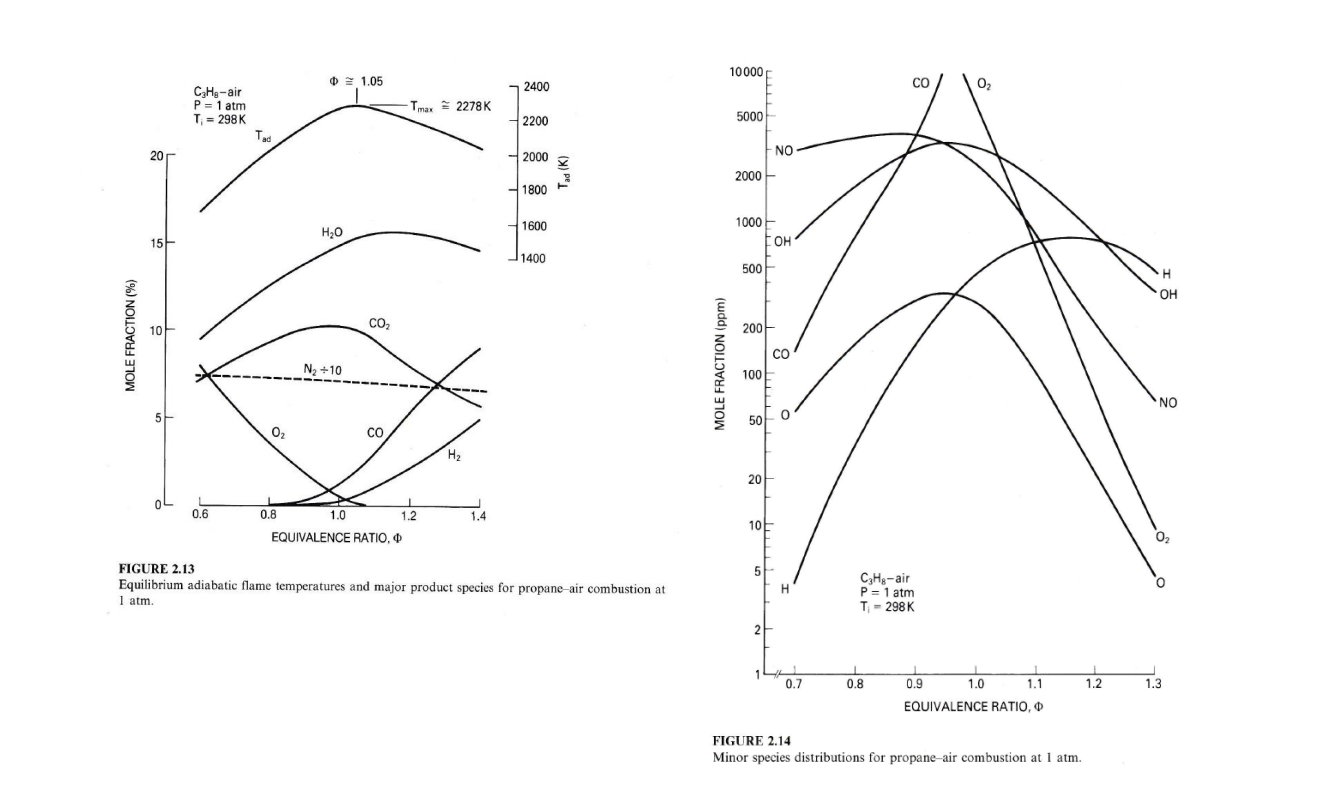
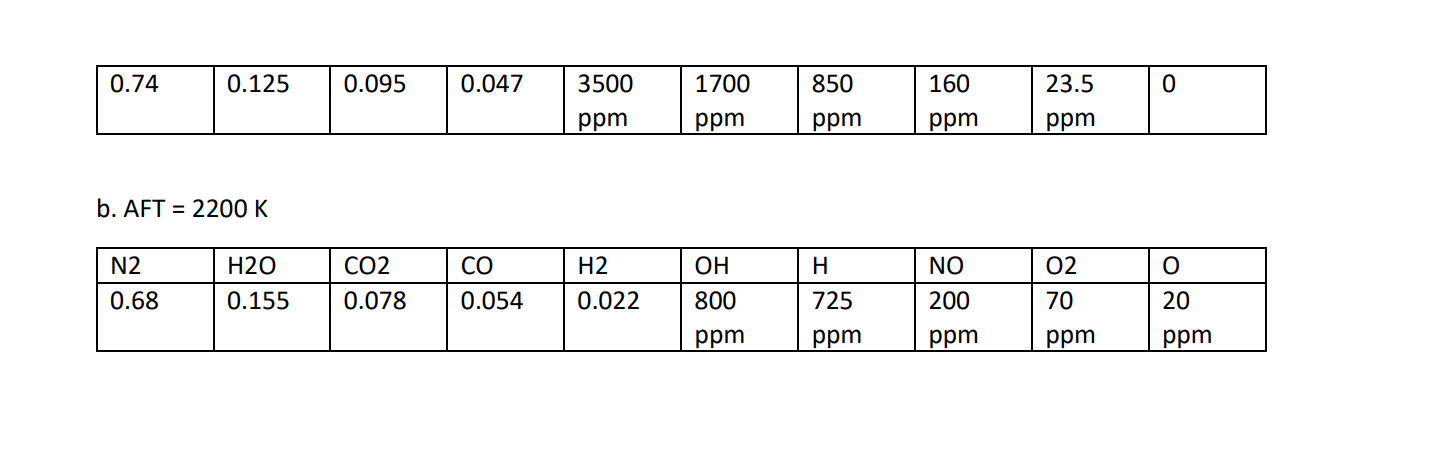
8. For the conditions listed below, rank from highest to lowest, the mole fractions xi = N;/Ntot of i= CO2, CO, H20, H2, OH, 02, 0, N2, NO and N for a. propane-air constant pressure combustion at their AFT with o=0.8 (fuel lean) b. same as a., but for o=1.2 (fuel rich) Use the following supplemental data as needed: 01.05 10000 2400 CO 02 CH,-air P = 1 atm T = 298K Tmax 2278K 2200 5000 Tad 20 2000 NO Tad (K) 2000 1800 1600 1000 H2O 154 OH 1400 500 OH CO2 200 MOLE FRACTION (%) CO Ny +10 MOLE FRACTION (ppm) 100 NO 500 O2 , H2 20 0.6 0.8 1.2 1.4 1.0 EQUIVALENCE RATIO, 10 5 5 FIGURE 2.13 Equilibrium adiabatic flame temperatures and major product species for propane-air combustion at 1 atm. . H C3H8-air P = 1 atm T-298 K 2 0.7 0.8 1.2 1.3 0.9 1.0 1.1 EQUIVALENCE RATIO, FIGURE 2.14 Minor species distributions for propane-air combustion at 1 atm. Ans. a. AFT = 2020 K | | N2 H20 CO2 02 NO CO 0 H H2 0.74 0.125 0.095 0.047 3500 1700 850 160 23.5 0 ppm ppm ppm ppm ppm b. AFT = 2200 K N2 H20 CO H2 o CO2 0.078 OH 800 . 725 NO 200 02 70 0.68 0.155 0.054 0.022 20 ppm ppm ppm ppm ppm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


