Answered step by step
Verified Expert Solution
Question
1 Approved Answer
this is the third time i've asked this question now, but still nobody has answered with an actual explanation on how to solve for this
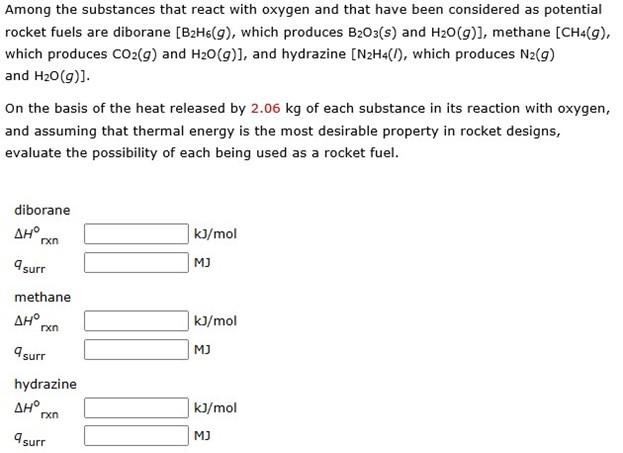
this is the third time i've asked this question now, but still nobody has answered with an actual explanation on how to solve for this problem and i desperately need the help solving it.
Among the substances that react with oxygen and that have been considered as potential rocket fuels are diborane [B2H6(g), which produces B2O3(s) and H2O(g)], methane [CH4(g), which produces CO2(g) and H2O(g)], and hydrazine [N2H4(I), which produces N2(g) and H2O(g)] On the basis of the heat released by 2.06kg of each substance in its reaction with oxygen, and assuming that thermal energy is the most desirable property in rocket designs, evaluate the possibility of each being used as a rocket fuelStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


