Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. (25 pts.) As an extension of #2, consider tropolone (1 below). Tropolone is a compound that can act as an acid by donating
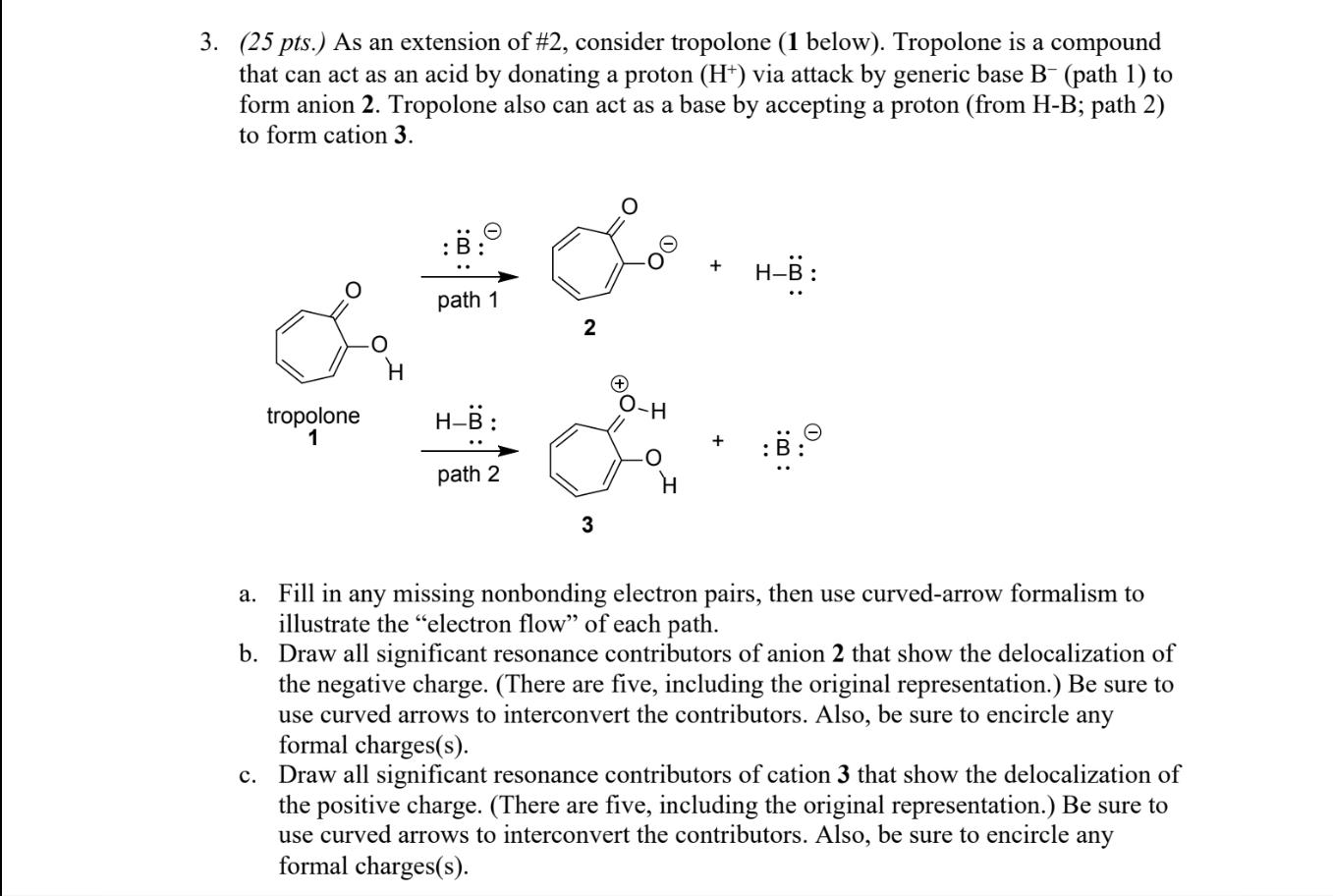
3. (25 pts.) As an extension of #2, consider tropolone (1 below). Tropolone is a compound that can act as an acid by donating a proton (H+) via attack by generic base B- (path 1) to form anion 2. Tropolone also can act as a base by accepting a proton (from H-B; path 2) to form cation 3. tropolone 1 O : path 1 H-B: path 2 2 3 + H-B : a. Fill in any missing nonbonding electron pairs, then use curved-arrow formalism to illustrate the "electron flow" of each path. b. Draw all significant resonance contributors of anion 2 that show the delocalization of the negative charge. (There are five, including the original representation.) Be sure to use curved arrows to interconvert the contributors. Also, be sure to encircle any formal charges(s). c. Draw all significant resonance contributors of cation 3 that show the delocalization of the positive charge. (There are five, including the original representation.) Be sure to use curved arrows to interconvert the contributors. Also, be sure to encircle any formal charges(s).
Step by Step Solution
★★★★★
3.45 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1
F H c 0 3 Rill in any missing nonbonding election pair The...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


