Answered step by step
Verified Expert Solution
Question
1 Approved Answer
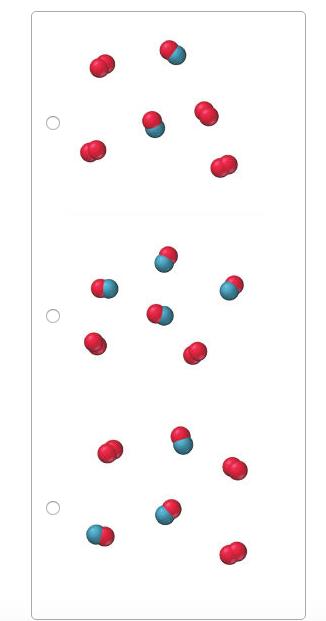
This reaction was experimentally determined to be first order with respect to O2 and second order with respect to NO. O2(g)+2NO(g)?2NO2(g) These diagrams represent reaction
This reaction was experimentally determined to be first order with respect to O2 and second order with respect to NO. O2(g)+2NO(g)?2NO2(g) These diagrams represent reaction mixtures in which the number of each type of molecule represents its relative initial concentration.
Which mixture has the fastest initial rate?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Required solution A...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
635e0ab56938d_180953.pdf
180 KBs PDF File
635e0ab56938d_180953.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


