Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Through much of your undergraduate work, you make the assumption that gases behave at or close to ideal. This assumption is normally fine and
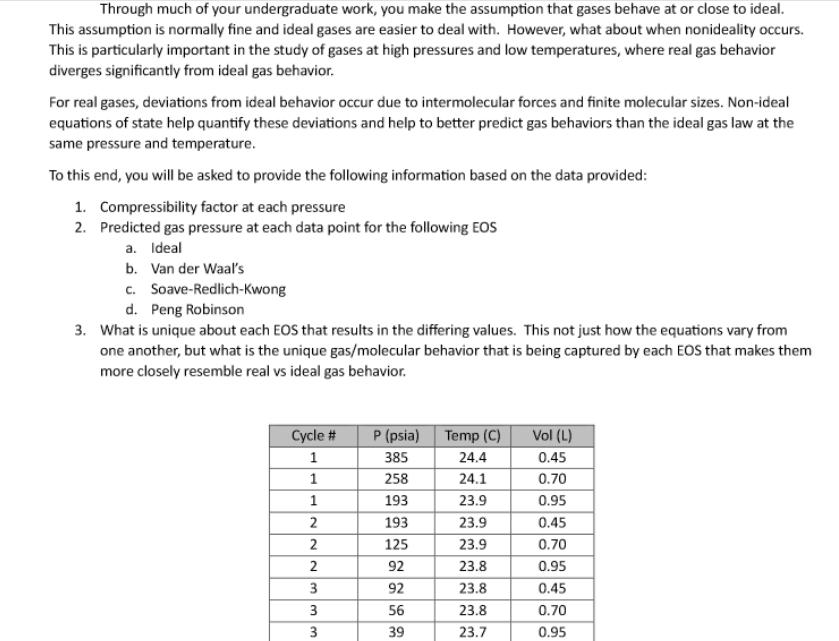
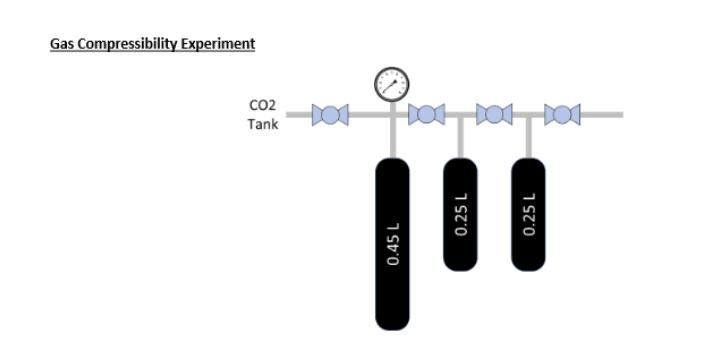
Through much of your undergraduate work, you make the assumption that gases behave at or close to ideal. This assumption is normally fine and ideal gases are easier to deal with. However, what about when nonideality occurs. This is particularly important in the study of gases at high pressures and low temperatures, where real gas behavior diverges significantly from ideal gas behavior. For real gases, deviations from ideal behavior occur due to intermolecular forces and finite molecular sizes. Non-ideal equations of state help quantify these deviations and help to better predict gas behaviors than the ideal gas law at the same pressure and temperature. To this end, you will be asked to provide the following information based on the data provided: 1. Compressibility factor at each pressure 2. Predicted gas pressure at each data point for the following EOS a. Ideal b. Van der Waal's c. Soave-Redlich-Kwong d. Peng Robinson 3. What is unique about each EOS that results in the differing values. This not just how the equations vary from one another, but what is the unique gas/molecular behavior that is being captured by each EOS that makes them more closely resemble real vs ideal gas behavior. Cycle # P (psia) Temp (C) Vol (L) 1 385 24.4 0.45 1 258 24.1 0.70 1 193 23.9 0.95 2 193 23.9 0.45 2 125 23.9 0.70 2 92 23.8 0.95 3 92 23.8 0.45 3 56 23.8 0.70 3 39 23.7 0.95 Gas Compressibility Experiment 0.45 L CO2 Tank 101 101 101 0.25 L 0.25 L
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


