Answered step by step
Verified Expert Solution
Question
1 Approved Answer
thx a) Determine the change in e.m. f., when we add to the cell a 0.2mol/kg solution of KF. (Consider that all electrolytes are fully
thx 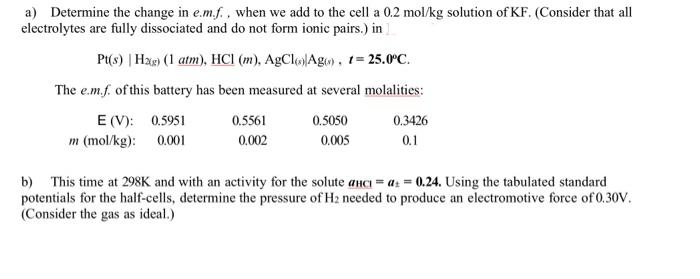
a) Determine the change in e.m. f., when we add to the cell a 0.2mol/kg solution of KF. (Consider that all electrolytes are fully dissociated and do not form ionic pairs.) in Pt(s)H2g)(1atm),HCl(m),AgCl(s)Ag(s),t=25.0C. The e.m.f. of this battery has been measured at several molalities: b) This time at 298K and with an activity for the solute aHCl=at=0.24. Using the tabulated standard potentials for the half-cells, determine the pressure of H2 needed to produce an electromotive force of 0.30V. (Consider the gas as ideal.) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


