Answered step by step
Verified Expert Solution
Question
1 Approved Answer
tipe the answer please Ahot solution containing 1000 kg of MgSO4 and water having a concentration of 30 w MgSO4 is cooled to 280.8K, where
tipe the answer please 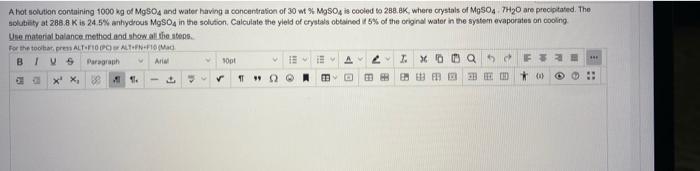
Ahot solution containing 1000 kg of MgSO4 and water having a concentration of 30 w MgSO4 is cooled to 280.8K, where crystals of MgSO4.7H20 are precipitated. The solubility at 288.8K is 24.5% anhydrous MgSO4 in the solution Calculate the yield of crystals obtained 5% of the original water in the system evaporates on cooling Une material balance method and show all the stops For the topres ALTOPOATING BIVS Paragraph Arla SO EBAT. ( Q5F32 X X, 38 14. DE 33 ) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


