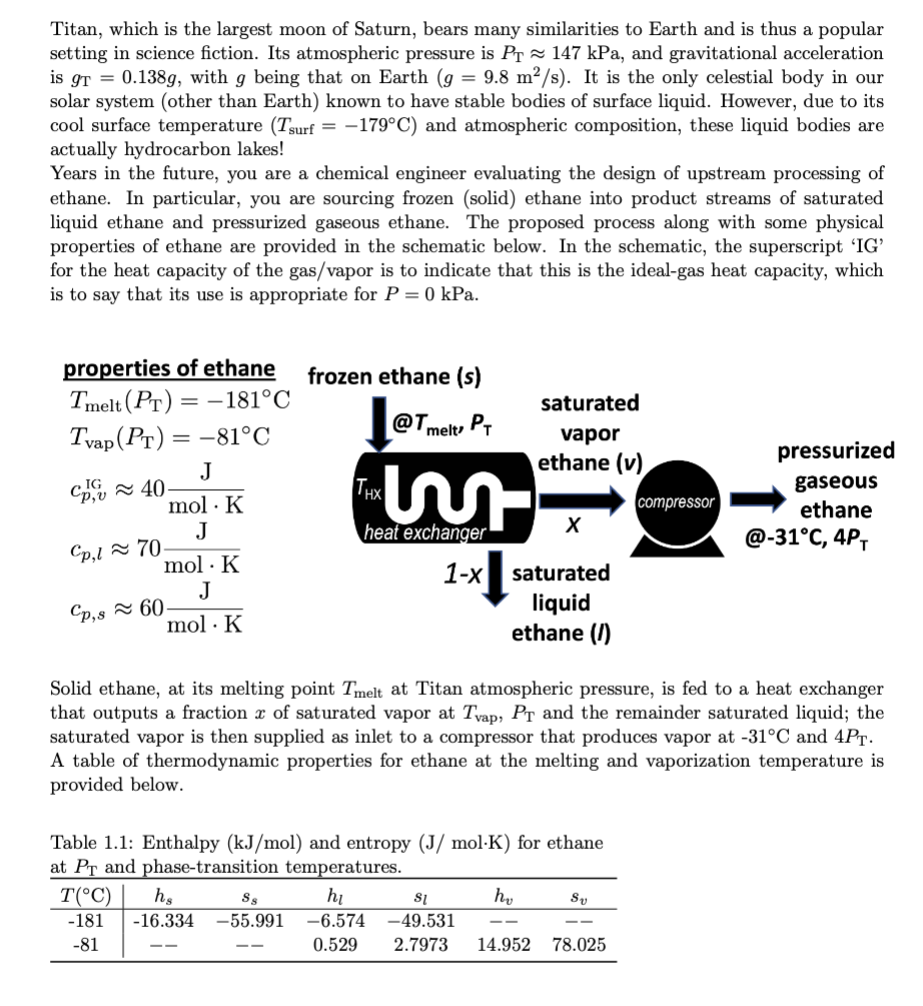
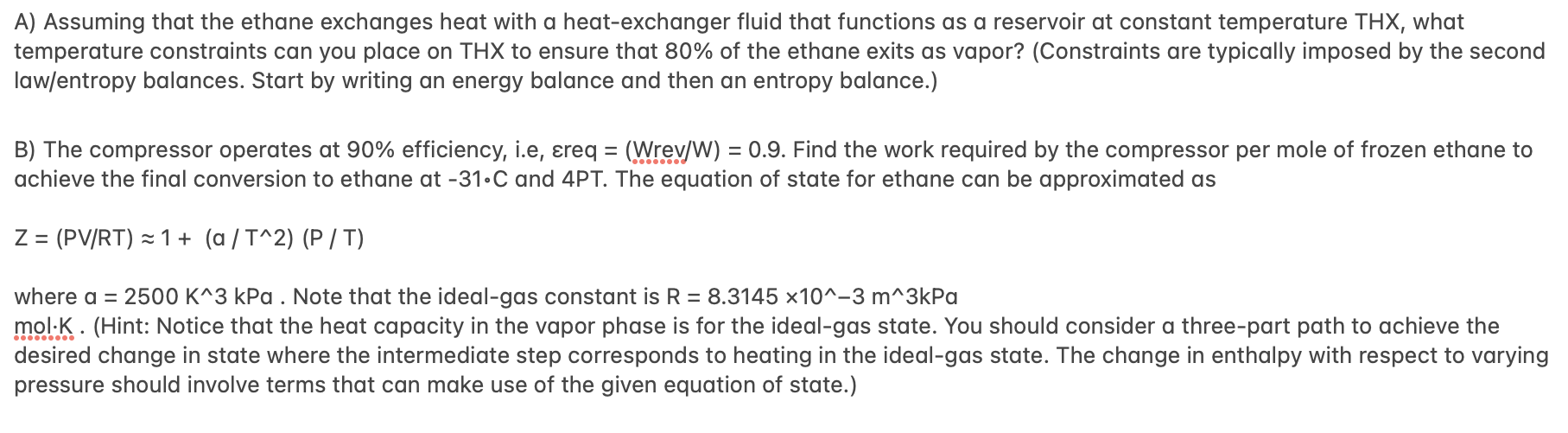
Titan, which is the largest moon of Saturn, bears many similarities to Earth and is thus a popular setting in science fiction. Its atmospheric pressure is PT147kPa, and gravitational acceleration is gT=0.138g, with g being that on Earth (g=9.8m2/s). It is the only celestial body in our solar system (other than Earth) known to have stable bodies of surface liquid. However, due to its cool surface temperature (Tsurf=179C) and atmospheric composition, these liquid bodies are actually hydrocarbon lakes! Years in the future, you are a chemical engineer evaluating the design of upstream processing of ethane. In particular, you are sourcing frozen (solid) ethane into product streams of saturated liquid ethane and pressurized gaseous ethane. The proposed process along with some physical properties of ethane are provided in the schematic below. In the schematic, the superscript 'IG' for the heat capacity of the gas/vapor is to indicate that this is the ideal-gas heat capacity, which is to say that its use is appropriate for P=0kPa. propertiesofethaneTmelt(PT)=181CTvap(PT)=81Ccp,vIG40molKJcp,l70molKJcp,s60molKJ Solid ethane, at its melting point Tmelt at Titan atmospheric pressure, is fed to a heat exchanger that outputs a fraction x of saturated vapor at Tvap,PT and the remainder saturated liquid; the saturated vapor is then supplied as inlet to a compressor that produces vapor at 31C and 4PT. A table of thermodynamic properties for ethane at the melting and vaporization temperature is provided below. Table 1.1: Enthalpy (kJ/mol) and entropy (J/molK) for ethane at PT and phase-transition temperatures. A) Assuming that the ethane exchanges heat with a heat-exchanger fluid that functions as a reservoir at constant temperature THX, what temperature constraints can you place on THX to ensure that 80% of the ethane exits as vapor? (Constraints are typically imposed by the second law/entropy balances. Start by writing an energy balance and then an entropy balance.) B) The compressor operates at 90% efficiency, i.e, req =(Wrev/W)=0.9. Find the work required by the compressor per mole of frozen ethane to achieve the final conversion to ethane at 31C and 4PT. The equation of state for ethane can be approximated as Z=(PV/RT)1+(a/T2)(P/T) where a=2500K3kPa. Note that the ideal-gas constant is R=8.3145103m3kPa mol.:... (Hint: Notice that the heat capacity in the vapor phase is for the ideal-gas state. You should consider a three-part path to achieve the desired change in state where the intermediate step corresponds to heating in the ideal-gas state. The change in enthalpy with respect to varying pressure should involve terms that can make use of the given equation of state.)








