Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Title: Extraction of Copper Sulphate ( C u S O 4 ) from Pollution Control Waste Assignment Instructions: A waste material from pollution control, containing
Title: Extraction of Copper Sulphate from Pollution Control Waste
Assignment Instructions:
A waste material from pollution control, containing copper oxide, underwent a leaching process with sulfuric acid, resulting in a slurry with a pulp density of and mass fraction of solid equal to The concentration of in the solution phase of the slurry was measured to be This slurry underwent countercurrent washing in three stages as shown in Figure with water. The volume fraction of liquid in the slurry remained consistent throughout the process. The volumetric flow rate of the liquid used for washing was equal to the volume of wash water. How much wash water is required to ensure that the discharge slurry contains only of the original copper content? The solids in the slurry have a density of
Based on your findings complete the table provided below and draw a graph showing the effect of the water flow rate on number of stages and the recovery of You need to draw a graph in which axis represents the flow rate of the wash water or concentration of in slurry and axis shows the number of stages.
Data. The approximate specific gravity of solutions is: of solution where is the mass fraction of
Hint: use of feed slurry as your basis.
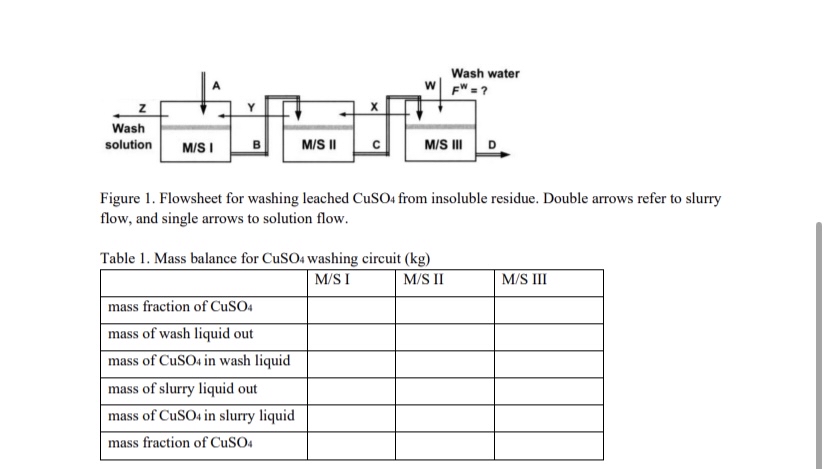
Figure Flowsheet for washing leached from insoluble residue. Double arrows refer to slurry flow, and single arrows to solution flow.
Table Mass balance for washing circuit
tableMS I,MS IIMS IIImass fraction of CuSO mass of wash liquid out,,,mass of in wash liquid,,,mass of slurry liquid out,,,mass of CuSO in slurry liquid,,,mass fraction of CuSO
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


