Answered step by step
Verified Expert Solution
Question
1 Approved Answer
To construct the galvanic cell illustrated above, the salt bridge was prepared by soaking a piece of cotton in 5.0MNaNO3(aq) before placing it inside the
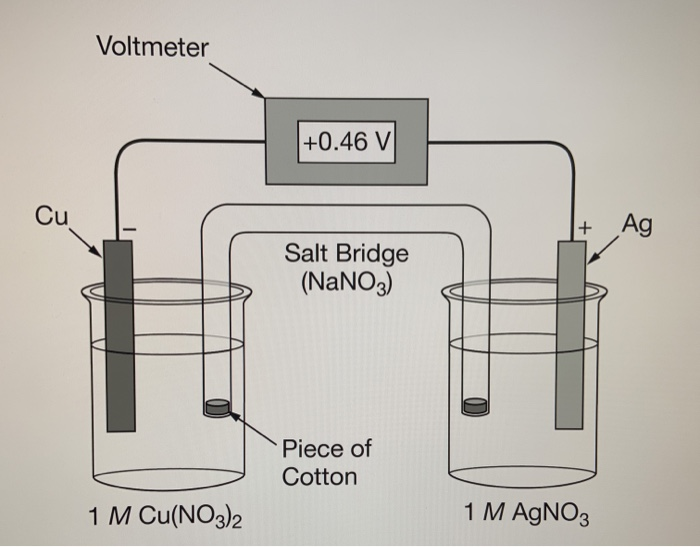
To construct the galvanic cell illustrated above, the salt bridge was prepared by soaking a piece of cotton in 5.0MNaNO3(aq) before placing it inside the U-shaped tube filled with distilled water. If the cotton was soaked in distilled water by mistake, which of the following best explains how the operation of the cell would be affected?
A. The operation of the cell is not affected because neither Na+(aq) nor NO3?(aq) is involved in the redox reaction that takes place.
B. The operation of the cell generates a higher potential because there are fewer ions in the solution, making the reaction more thermodynamically favored.
C. The cell will operate for a much longer time because the flow of electrons through the circuit will eventually be reversed.
D. The cell would not operate because a current could not be conducted between the half-cells.
Cu Voltmeter 1 M Cu(NO3)2 +0.46 V Salt Bridge (NaNO3) Piece of Cotton + Ag 1 M AgNO3
Step by Step Solution
★★★★★
3.42 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
Salt bridge is a device which is necessary to maintain the electrical neut...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


