Answered step by step
Verified Expert Solution
Question
1 Approved Answer
To heat 1 cup of water (250 cm) to make coffee, you place an electric heating element in the cup. As the water temperature
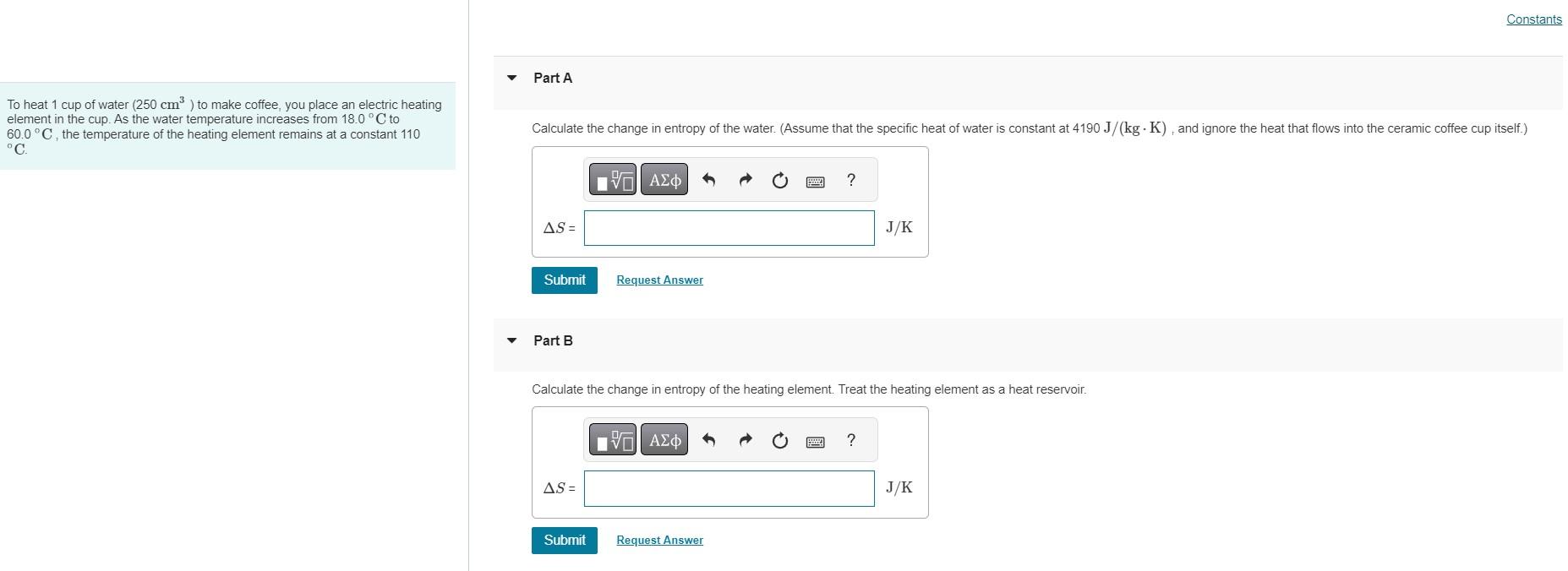
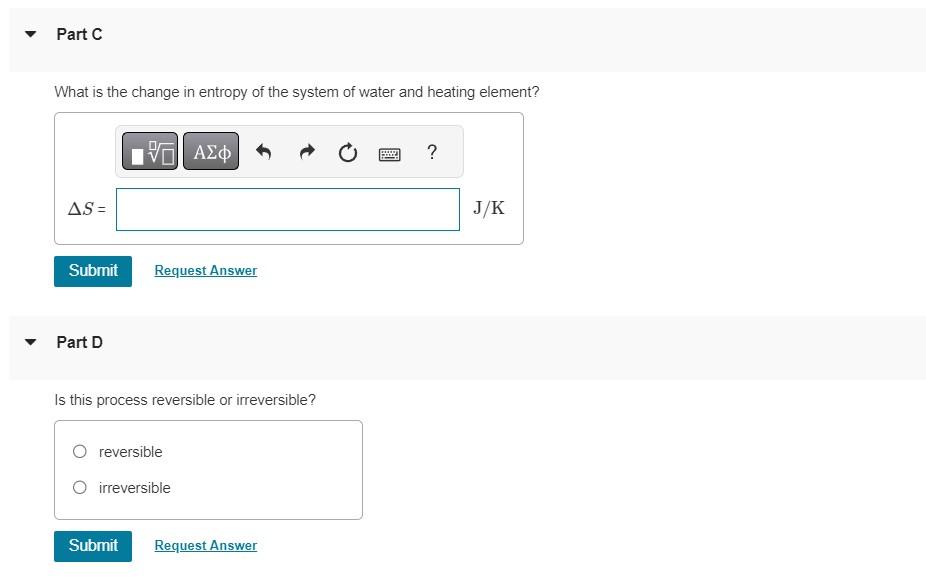
To heat 1 cup of water (250 cm) to make coffee, you place an electric heating element in the cup. As the water temperature increases from 18.0C to 60.0 C, the temperature of the heating element remains at a constant 110 C. Part A Constants Calculate the change in entropy of the water. (Assume that the specific heat of water is constant at 4190 J/(kg K), and ignore the heat that flows into the ceramic coffee cup itself.) AS= Submit Request Answer Part B ? J/K Calculate the change in entropy of the heating element. Treat the heating element as a heat reservoir. AS= Submit Request Answer ? J/K Part C What is the change in entropy of the system of water and heating element? AS= Submit Request Answer Part D Is this process reversible or irreversible? reversible O irreversible Submit Request Answer ? J/K
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


