Answered step by step
Verified Expert Solution
Question
1 Approved Answer
To how many significant figures should each answer be rounded? (6.626 x 10-34 J-s) (2.9979 108 m/s) 4.690 x 10-7 m equation A: After

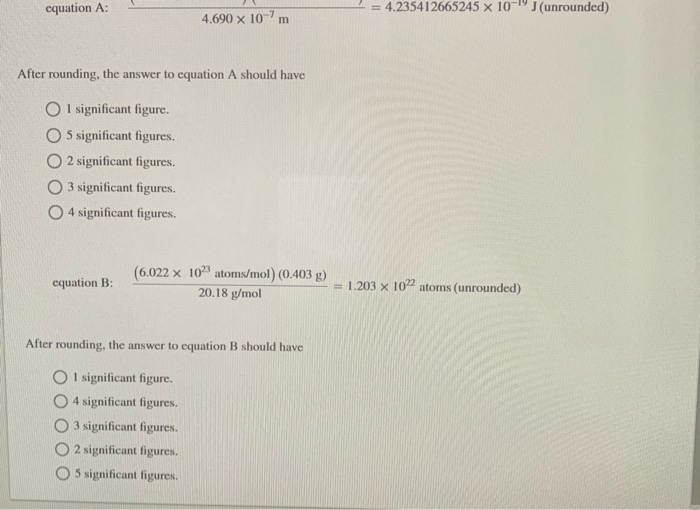
To how many significant figures should each answer be rounded? (6.626 x 10-34 J-s) (2.9979 108 m/s) 4.690 x 10-7 m equation A: After rounding, the answer to equation A should have 1 significant figure. 5 significant figures. 2 significant figures. 3 significant figures. O4 significant figures. equation B: (6.022 x 1023 atoms/mol) (0.403 g) 20.18 g/mol After rounding, the answer to equation B should have 1 significant figure. 4 significant figures. 3 significant figures. O2 significant figures. = 4.235412665245 x 10-19 J (unrounded) 1.203 x 1022 atoms (unrounded) equation A: After rounding, the answer to equation A should have O1 significant figure. 5 significant figures. 2 significant figures. O3 significant figures. O4 significant figures. 4.690 x 107 m equation B: (6.022 x 1023 atoms/mol) (0.403 g) 20.18 g/mol After rounding, the answer to equation B should have O1 significant figure. O4 significant figures. O3 significant figures. O2 significant figures. O5 significant figures. 4.235412665245 x 10-19 J(unrounded) = 1.203 x 1022 atoms (unrounded)
Step by Step Solution
★★★★★
3.46 Rating (143 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


