Answered step by step
Verified Expert Solution
Question
1 Approved Answer
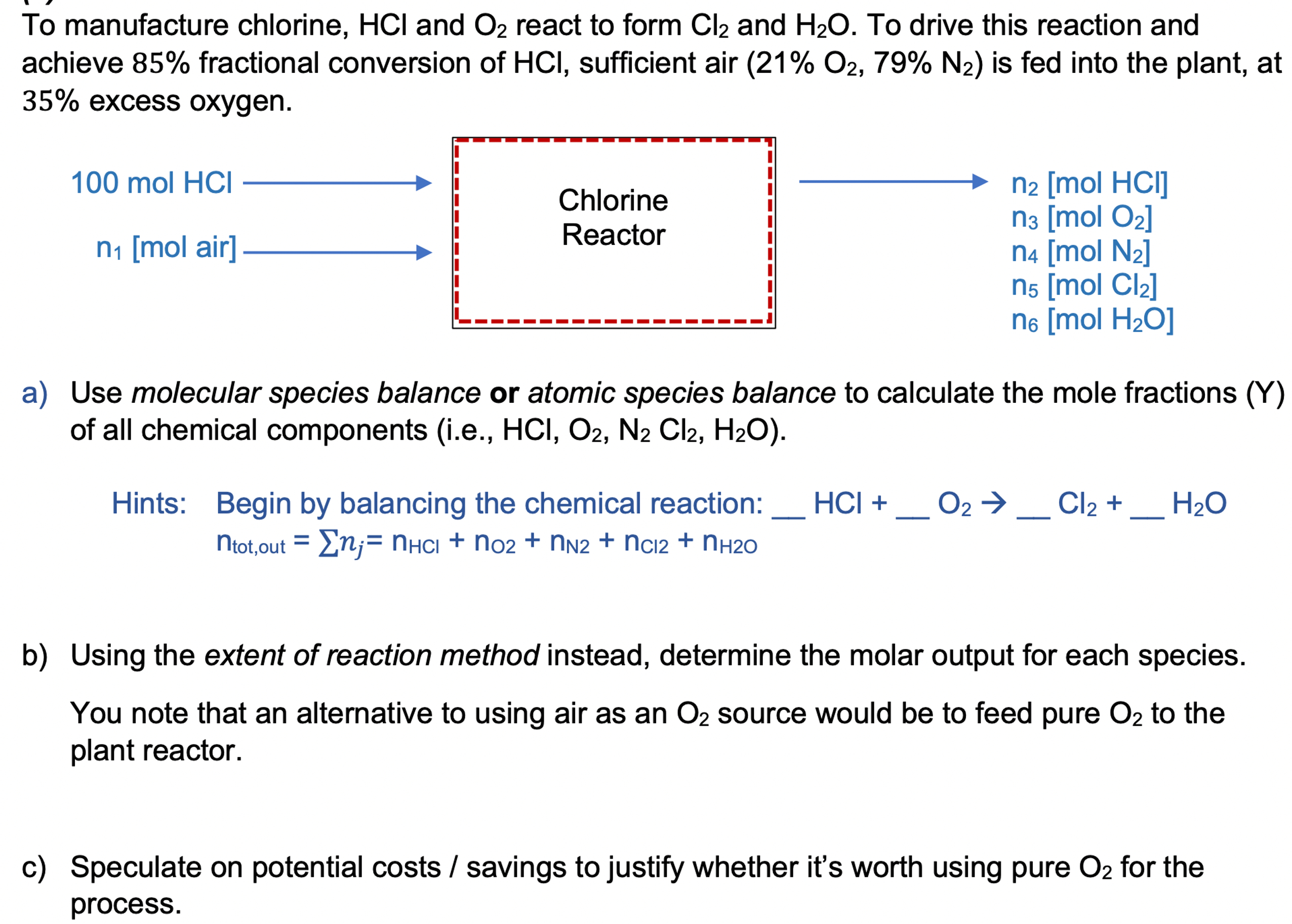
To manufacture chlorine, H C l and O 2 react to form C l 2 and H 2 O . To drive this reaction and
To manufacture chlorine, and react to form and To drive this reaction and
achieve fractional conversion of sufficient air is fed into the plant, at
excess oxygen.
a Use molecular species balance or atomic species balance to calculate the mole fractions Y
of all chemical components ie
Hints: Begin by balancing the chemical reaction:
b Using the extent of reaction method instead, determine the molar output for each species.
You note that an alternative to using air as an source would be to feed pure to the
plant reactor.
c Speculate on potential costs savings to justify whether it's worth using pure for the
process.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


