Question
To measure the quantity of MnCl dissolved in an aqueous solution, it was completely converted to KMnO4 using the reaction, MnCl + KS2O8 +
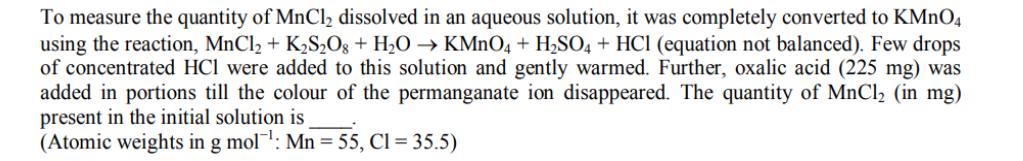
To measure the quantity of MnCl dissolved in an aqueous solution, it was completely converted to KMnO4 using the reaction, MnCl + KS2O8 + HO KMnO4 + HSO4 + HCl (equation not balanced). Few drops of concentrated HCl were added to this solution and gently warmed. Further, oxalic acid (225 mg) was added in portions till the colour of the permanganate ion disappeared. The quantity of MnCl (in mg) present in the initial solution is (Atomic weights in g mol: Mn = 55, Cl = 35.5)
Step by Step Solution
3.55 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Essential Statistics In Business And Economics
Authors: David Doane, Lori Seward
3rd Edition
1260239500, 978-1260239508
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



