Answered step by step
Verified Expert Solution
Question
1 Approved Answer
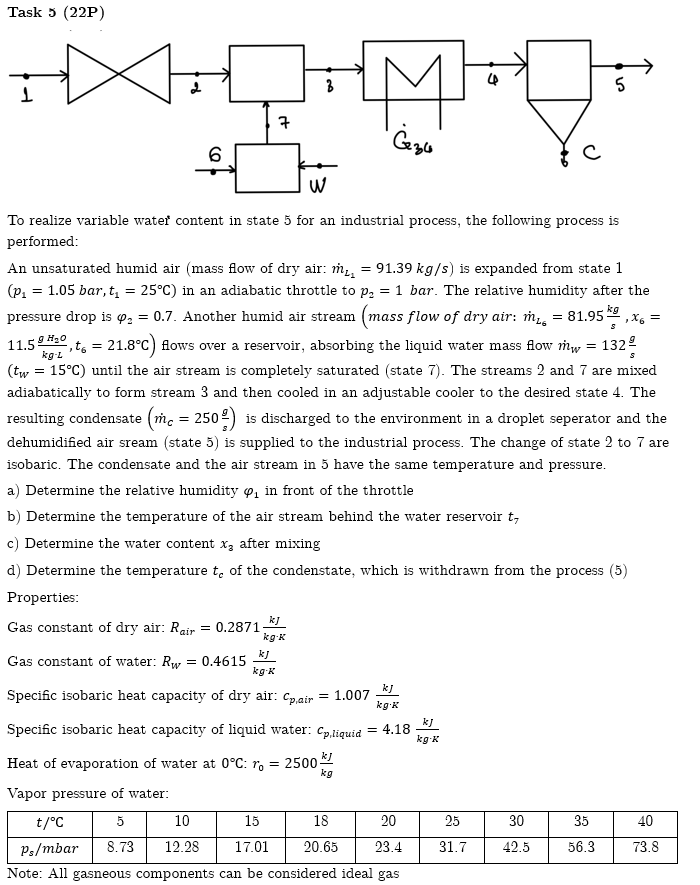
To realize variable water content in state 5 for an industrial process, the following process is performed: An unsaturated humid air ( mass flow of
To realize variable water content in state for an industrial process, the following process is
performed:
An unsaturated humid air mass flow of dry air: is expanded from state
in an adiabatic throttle to The relative humidity after the
pressure drop is Another humid air stream mass flow of dry air:
flows over a reservoir, absorbing the liquid water mass flow
until the air stream is completely saturated state The streams and are mixed
adiabatically to form stream and then cooled in an adjustable cooler to the desired state The
resulting condensate is discharged to the environment in a droplet seperator and the
dehumidified air sream state is supplied to the industrial process. The change of state to are
isobaric. The condensate and the air stream in have the same temperature and pressure.
a Determine the relative humidity in front of the throttle
b Determine the temperature of the air stream behind the water reservoir
c Determine the water content after mixing
d Determine the temperature of the condenstate, which is withdrawn from the process
Properties:
Gas constant of dry air:
Gas constant of water:
Specific isobaric heat capacity of dry air:
Specific isobaric heat capacity of liquid water:
Heat of evaporation of water at :
Vapor pressure of water:
Note: All gasneous components can be considered ideal gas
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


