Answered step by step
Verified Expert Solution
Question
1 Approved Answer
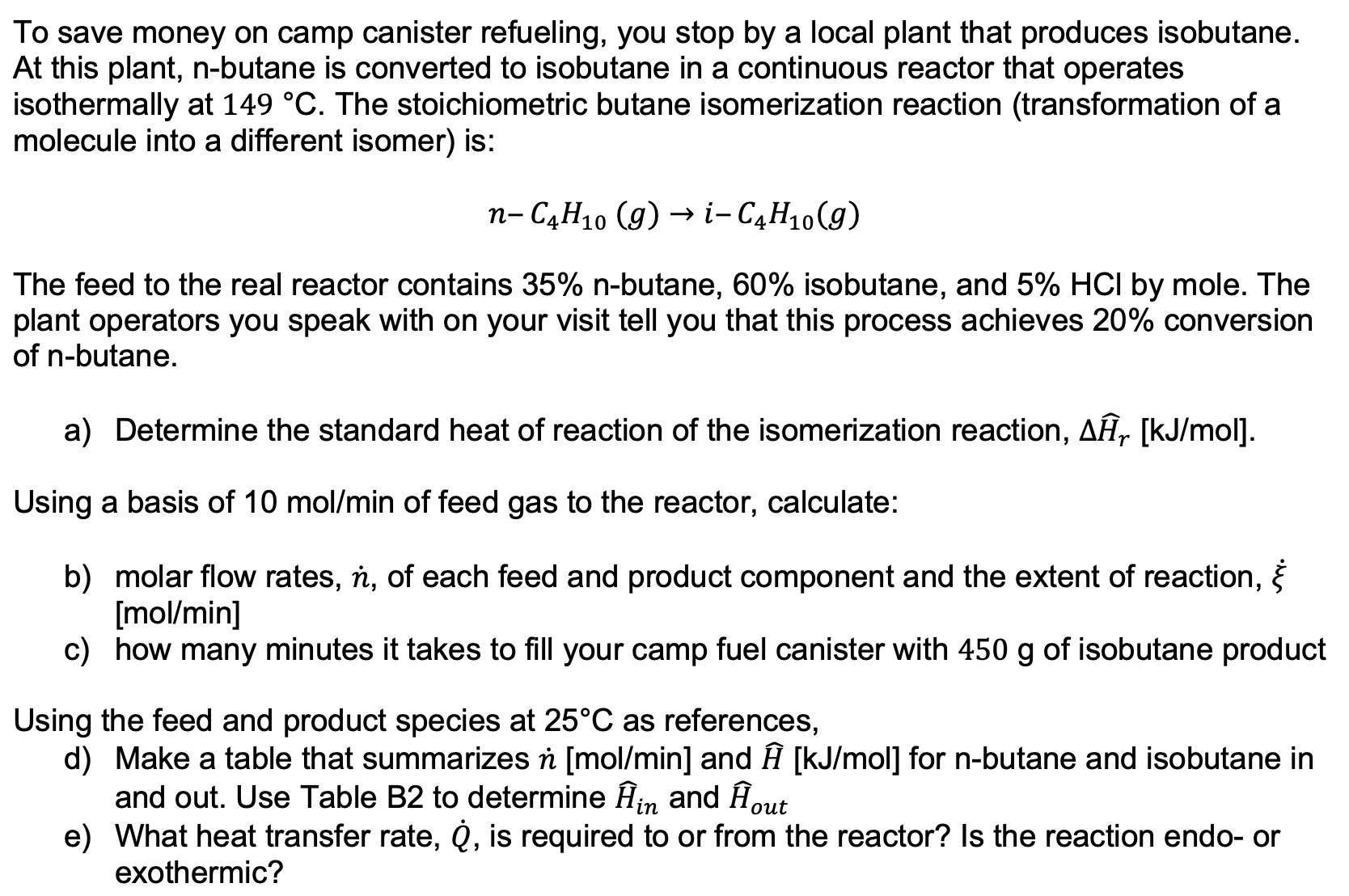
To save money on camp canister refueling, you stop by a local plant that produces isobutane. At this plant, n - butane is converted to
To save money on camp canister refueling, you stop by a local plant that produces isobutane.
At this plant, butane is converted to isobutane in a continuous reactor that operates
isothermally at The stoichiometric butane isomerization reaction transformation of a
molecule into a different isomer is:
The feed to the real reactor contains nbutane, isobutane, and by mole. The
plant operators you speak with on your visit tell you that this process achieves conversion
of butane.
a Determine the standard heat of reaction of the isomerization reaction,
Using a basis of of feed gas to the reactor, calculate:
b molar flow rates, of each feed and product component and the extent of reaction,
c how many minutes it takes to fill your camp fuel canister with of isobutane product
Using the feed and product species at as references,
d Make a table that summarizes and widehat for butane and isobutane in
and out. Use Table B to determine widehat and widehat
e What heat transfer rate, is required to or from the reactor? Is the reaction endo or
exothermic?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


