Question
Total Metal Contents of samples Sample ID Sample 1: Crushed CRT funnel glass Sample 2: Crushed CRT panel glass TCLP results CRT funnel glass
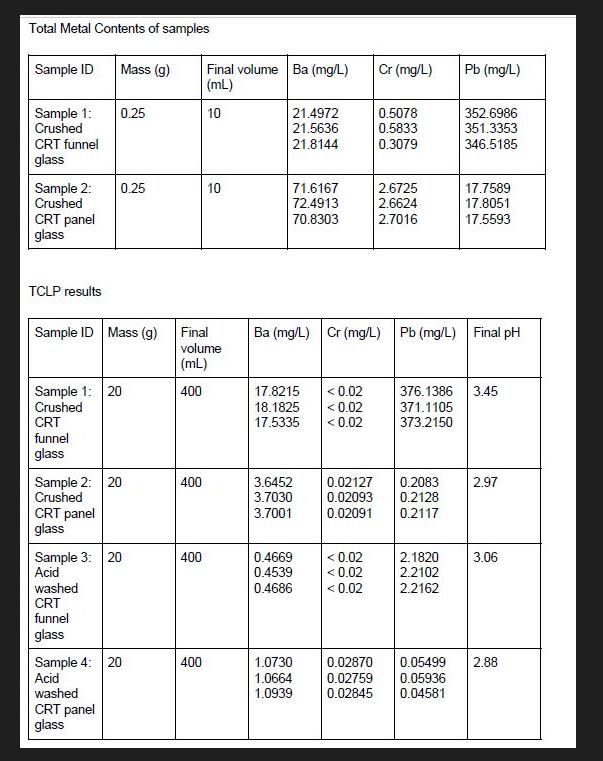
Total Metal Contents of samples Sample ID Sample 1: Crushed CRT funnel glass Sample 2: Crushed CRT panel glass TCLP results CRT funnel glass Sample ID Mass (g) Mass (g) Sample 1: 20 Crushed CRT panel glass 0.25 0.25 Sample 2: 20 Crushed washed CRT funnel glass Sample 3: 20 Acid washed CRT panel glass Sample 4: 20 Acid 400 Final volume (mL) 400 400 Final volume Ba (mg/L) (mL) 10 400 10 21.4972 21.5636 21.8144 71.6167 72.4913 70.8303 17.8215
Step by Step Solution
3.54 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
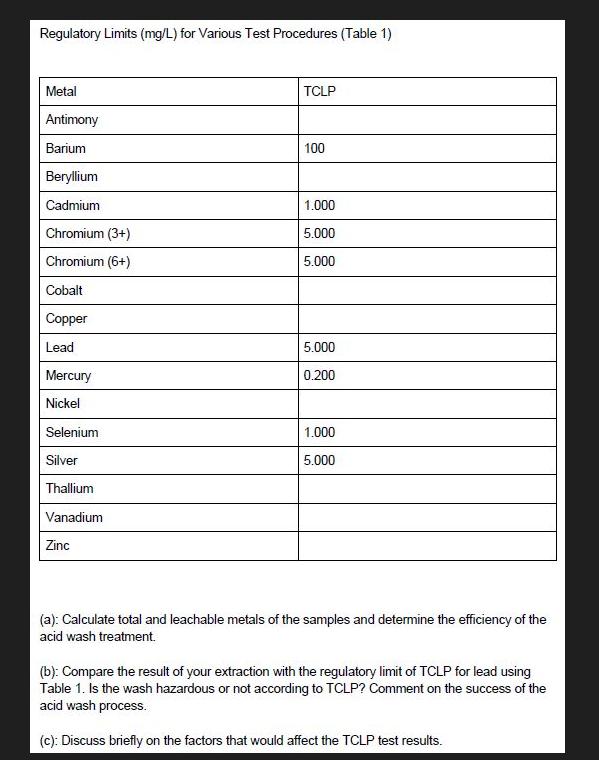
Calculate total and leachable metals of the samples and determine the efficiency of the acid wash treatment Sample 1 Crushed CRT funnel glass Total metal content Ba 3526986 mgL Cr 05833 mgL Pb 03079 m...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Quantitative Chemical Analysis
Authors: Daniel C. Harris
8th edition
1429218150, 978-1429218153
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



