Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Emission Trace amounts of rare elements are found within groundwater and are of interest to geochemists. Europium and terbium are lanthanide-series elements that can
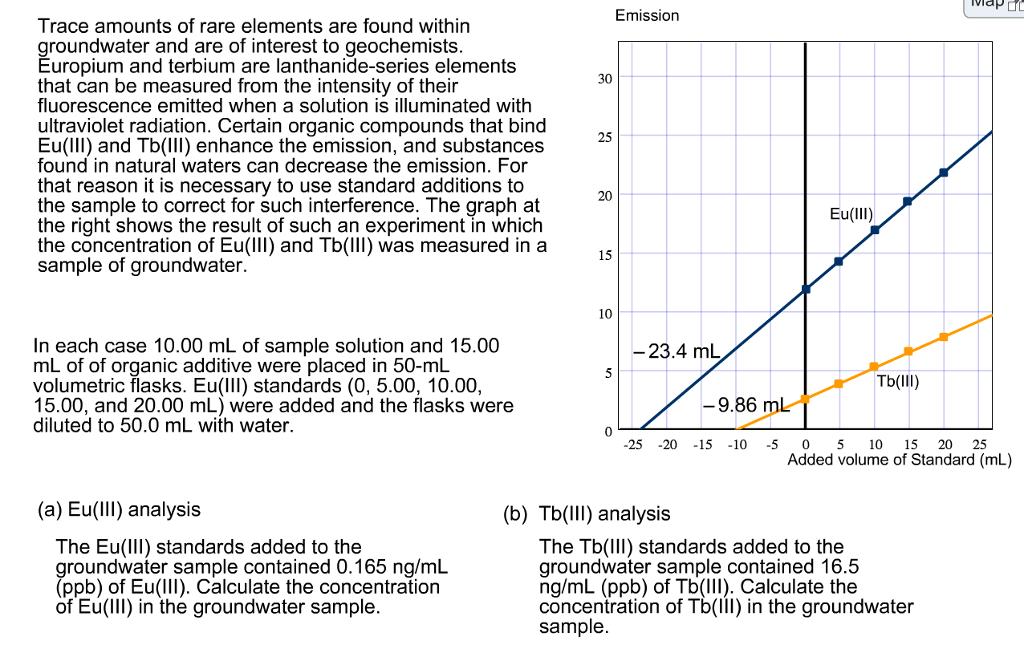
Emission Trace amounts of rare elements are found within groundwater and are of interest to geochemists. Europium and terbium are lanthanide-series elements that can be measured from the intensity of their fluorescence emitted when a solution is illuminated with ultraviolet radiation. Certain organic compounds that bind Eu(III) and Tb(III) enhance the emission, and substances found in natural waters can decrease the emission. For that reason it is necessary to use standard additions to the sample to correct for such interference. The graph at the right shows the result of such an experiment in which the concentration of Eu(llI) and Tb(III) was measured in a sample of groundwater." 30 25 20 Eu(III) 15 10 In each case 10.00 mL of sample solution and 15.00 mL of of organic additive were placed in 50-mL volumetric flasks. Eu(III) standards (0, 5.00, 10.00, 15.00, and 20.00 mL) were added and the flasks were diluted to 50.0 mL with water. - 23.4 mL 5 Tb(III) -9.86 mL -25 -20 -15 10 15 20 Added volume of Standard (mL) -10 -5 25 (a) Eu(III) analysis (b) Tb(1II) analysis The Eu(lII) standards added to the groundwater sample contained 0.165 ng/mL (ppb) of Eu(lII). Calculate the concentration of Eu(III) in the groundwater sample. The Tb(II) standards added to the groundwater sample contained 16.5 ng/mL (ppb) of Tb(III). Calculate the concentration of Tb(lI) in the groundwater sample.
Step by Step Solution
★★★★★
3.45 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


