Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Treating air as an ideal gas of diatomic molecules, calculate how much heat is required to raise the temperature of the air in a
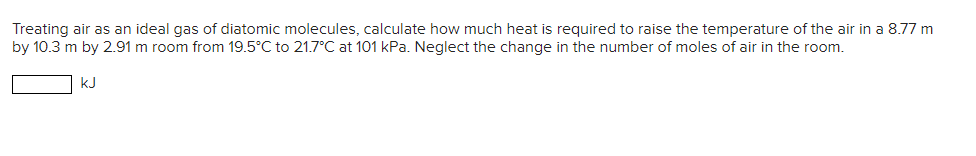
Treating air as an ideal gas of diatomic molecules, calculate how much heat is required to raise the temperature of the air in a 8.77 m by 10.3 m by 2.91 m room from 19.5C to 21.7C at 101 kPa. Neglect the change in the number of moles of air in the room. KJ
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To calculate the heat required to raise the temperature of air in the room we will use the formula Q ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Document Format ( 2 attachments)
663e47fdb5d63_958747.pdf
180 KBs PDF File
663e47fdb5d63_958747.docx
120 KBs Word File
Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


