Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Trial Trial 1 Calculation (ML) Measurements Final (degree Celsius) 250+250 20c 98c 56c Trial 2 200+400 20c 98c 72c Trial3 300+150 15c 90c 41c
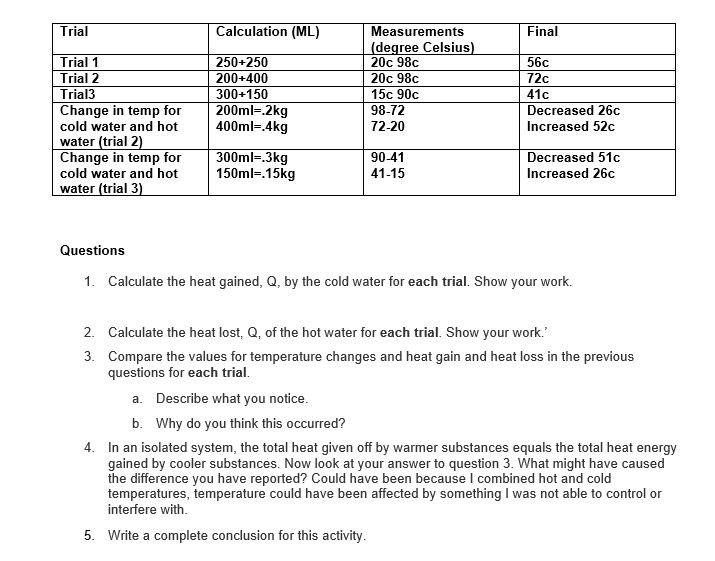
Trial Trial 1 Calculation (ML) Measurements Final (degree Celsius) 250+250 20c 98c 56c Trial 2 200+400 20c 98c 72c Trial3 300+150 15c 90c 41c Change in temp for 200ml-.2kg 98-72 cold water and hot 400ml=.4kg 72-20 Decreased 26c Increased 52c water (trial 2) Change in temp for 300ml=.3kg 90-41 Decreased 51c cold water and hot 150ml=.15kg 41-15 Increased 26c water (trial 3) Questions 1. Calculate the heat gained, Q, by the cold water for each trial. Show your work. 2. Calculate the heat lost, Q, of the hot water for each trial. Show your work.' 3. Compare the values for temperature changes and heat gain and heat loss in the previous questions for each trial. a. Describe what you notice. b. Why do you think this occurred? 4. In an isolated system, the total heat given off by warmer substances equals the total heat energy gained by cooler substances. Now look at your answer to question 3. What might have caused the difference you have reported? Could have been because I combined hot and cold temperatures, temperature could have been affected by something I was not able to control or interfere with. 5. Write a complete conclusion for this activity.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


